December 23, 2020 at 6:36 am | Updated March 15, 2022 at 11:44 am | 7 min read
Scientists are trying to work out how to capitalize on the increase in atmospheric carbon dioxide to increase crop yield. Leaf area is one of the main morphological features that is affected and is instrumental in influencing carbon assimilation. Though leaves are easily accessible, measurements of leaf area must be precise and non-destructive. New laser-based portable scientific tools, like the CI-203 Handheld Laser Leaf Area Meter, make leaf area measurement easy—even in the field.
Checking Soybean Response to Increased Atmospheric Carbon
High levels of atmospheric carbon dioxide (CO2) are leading to climate change and are expected to reach 800 μL L-1 by 2100. Notwithstanding the serious negative implications of such a change, these increases in carbon dioxide could boost crop yield.
Experiments have shown that a carbon dioxide increase can improve soybean yield in greenhouses. However, there are various other factors that influence the extent of the increase, such as the cultivar and other agricultural management practices.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
One of the physiological features expected to be affected is leaf area. In turn, changes in leaf area alter canopy structure and carbon assimilation, which directly influences biomass production and grain yield. The vegetative and reproductive stages of soybean overlap, so the seeds that act as a carbon sink can benefit from carbon assimilation by leaves in their immediate vicinity.
The scientists—Jin, Li, Liu, Wang, Tang, Yu, Wang, and Herbert—decided to study the influence of the distribution of nodal leaf area on soybean yields. Increasing nitrogen supply to plants is important to help in the assimilation of increased carbon dioxide, but their concentrations can differ depending on soil type.
Nodal leaf area distribution was studied in two varieties: Suinong 14 and Dongsheng 7, grown in two Mollisol soils. The main treatment was two levels for carbon – eCO2, (580 ppm) and (380 ppm), and the experiment was conducted in open-top chambers. There were four replications of each test, so there were a total of 32 replicates.
Three soybean plants were grown in each of the 32 pots, and the leaf area measurements were taken on the following days:
- Day 68 at R4 (full pod setting)
- Day 85 at R5 (initial pod filling)
- Day 101 at R6 (full seed)
Leaf area at each node on the main axis and branch were measured to calculate leaf area duration (LAD) to get the number of days of green area in each of the 96 soybean plants. The formula used was
LAD = (Leaf area1 + Leaf area2) x (T2-T1)/2,
where Leaf area1 and Leaf area2 are the total leaf area measured on growth stages T1 and T2, respectively.
The scientists also counted the number of nodes and branches. This was then compared to total chlorophyll, leaf nitrogen, pod number, seed number, seed size, grain yield, and quality (protein %).
Challenging Requirements
Leaf area measurement has always played an important role in plant science and crop research, as leaves are involved in growth and photosynthesis.
Over the years, many methods have been developed to study leaf area, such as grid counting, gravimetric method, planimeter, regression equations, and image processing.
- Grid count is one of the simplest approaches. It requires only a grid paper and a pencil. The leaf is placed on the grid paper, the outline is traced, and the number of grids covered by leaves is counted.
- Gravimetric method also involves making a trace of the leaf, then cutting the outline to measure the weight of the paper cut-out with a paper of known area and weight, to deduce the area of the leaf indirectly.
Besides being destructive, both of these methods are laborious, time-consuming, and not suitable when a large number of leaves have to be measured, as in this experiment. The scientists wanted to measure 96 plants three times during the entire experiment, totalling 288 measurements.
- Planimeter measurement is not time-consuming but is not suitable for small leaves.
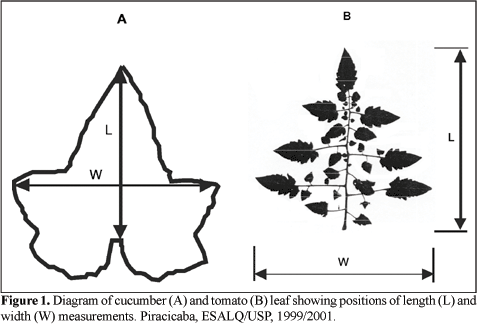
Figure 1: Linear Regression method involves measuring leaf length and width with a simple ruler, Blanco, and Folegatti, 2003 (Image credits: https://doi.org/10.1590/S0102-05362003000400019)
Other methods are non-destructive and accurate, but they are still time-consuming and involve several manual steps, such as the use of mathematical models and image processing.
- Mathematical models use manual measurements of the leaf length and width in regression models to calculate actual leaf area; see Figure 1.
- Image processing is non-destructive but involves image acquisition, pre-processing, segmentation, region filling, and area calculation. There are several methods of leaf area processing that also use transformation based on algorithms. Though non-destructive and precise, this method involves several steps and calculations that must be performed by the scientists to estimate the leaf area.
While these methods have the advantage of using simple everyday tools and are not expensive, using them requires meticulous labeling, time, and effort,
Given the number of leaves that Jin et al. wanted to measure, they needed a method that was accurate, easy, and, most importantly, non-destructive because LAD calculation requires the same leaves to be measured throughout the experiment without any damage.
Solution: The CI-203 Handheld Laser Leaf Area Meter
The scientists chose the CI-203 Handheld Laser Leaf Area Meter, manufactured by CID Bio-Science Inc.
The instrument is small and lightweight and can be operated with a single hand. The CI-203 is intuitive to use, and measurements are made by simply sweeping the measuring wand over the leaf.
The wand flattens curled leaves, and a low energy laser beam scans the leaf 500 times a second at a rate of 150 m/s. An optical sensor records the wand motion, and an onboard processing unit collects the data to measure the leaf length, width, area, and perimeter. When the motion stops, the device stops taking measurements. After each scan, the leaf outline is displayed on a built-in LCD screen.
Using pre-installed formulae, the device calculates shape ratio, shape factor, and void counts.
The device is versatile and can measure any leaf up to a width of 15 cm and a thickness of 1.4 cm, and it is suitable for soybean. In fact, it can be used for most crops and tree leaves that are not unusually large.
The CI-203 has an SD card that can store 15,000 individual readings, allowing the scientists to focus on their task at hand, rather than data-storage woes.
The device is shipped with the following included, and a conveyor attachment can be purchased separately for measuring especially large numbers of detatched leaves.
- Laser leaf area meter main unit
- SD Card
- Micro USB cable
- Battery charger
- Operating manual
- Hard-shell instrument carrying case
Benefits of Using a Leaf Meter
Compared to traditional methods, leaf area measurement with the CI-203 is easy, fast, and accurate. Moreover, hassle-free readings can be taken in the field.
An inbuilt GPS tags each measurement with its location, making it easy to track the changes in leaf area of individual leaves, which is an advantage in a time-series experiment, like Jin et al.’s study. Labeling on the plants or samples was also not necessary, saving more time in the process.
In addition, scientists benefited from digital data collection and storage. A WiFi USB helps in the easy and fast transfer of data to the computer. Moreover, leaf area measurement and data entry are happening simultaneously, saving the scientists time and effort and allowing them to immediately dive into statistical analysis.
Since the kit also has a rechargeable battery and charger, it is easy to charge the device at the end of the day.
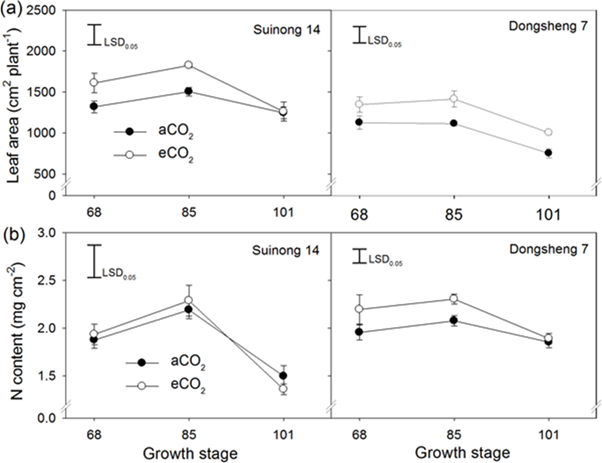
Figure 2: “The effect of elevated CO2 on leaf area (a) and N content per unit leaf area (b) during the period from the R4 (68 d), R5 (85 d) to R6 (101 d) stages. Two soybean cultivars, Suinong 14 and Dongsheng 7, were grown in Mollisols under ambient (aCO2) (380 ppm) and elevated CO2 (eCO2) (580 ppm),” Jin et al 2017. (Image credits: https://doi.org/10.1371/journal.pone.0176688.g003)
Outcome of the Experiment
Overall, there were more nodes and branches produced, which bore additional pods, leading to both higher levels of atmospheric carbon dioxide and an increase in yield.
This was attributed to an increase in leaf area between R4 to R6; see Figure 2. So, there were more nodes and higher leaf area in the upper canopy and less leaf area in the lower canopy, helping the plant to assimilate more carbon dioxide. The effect was higher—around 4.3%— between the R5 to R6 period than the R4 to R5 period. The leaf duration on branches was also longer by 2.4 times compared to the main stem.
The cultivars differed in their response, with greater yield increases happening in Dongsheng 7 than Suinong 14, which also corresponds to leaf nitrogen levels. Soil type did not influence the yield in this experiment, nor was there any change in seed protein content.
Leaf Area Measurements
With leaf area being key to crop performance and response to environmental factors, its use in experiments is likely to increase, especially in research driven by climate change. The importance of sophisticated methods of measurements, like the CI-203 Laser Leaf Area Meter, cannot be overstated. A single experiment can require hundreds of tests, and some need laboratory estimation. Having an instant measurement and recording of even part of the data set makes a scientist’s work easier and reduces the number of tasks they have to juggle during an experiment.
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature image courtesy of Sentinel Hub
Sources
Blanco, F. F., & Folegatti, M. V. (2003). A new method for estimating the leaf area index of cucumber and tomato plants. Horticultura Brasileira, 21(4), 666-669. doi:10.1590/s0102-05362003000400019
Chaudhary, P., Godara, S., Cheeran, A. N., & Chaudhari, A. K. (2012). Fast and Accurate Method for Leaf Area Measurement. International Journal of Computer Applications, 49(9), 22-25. doi:10.5120/7655-0757
Jin, J., Li, Y., Liu, X., Wang, G., Tang, C., Yu, Z., . . . Herbert, S. J. (2017). Elevated CO2 alters distribution of nodal leaf area and enhances nitrogen uptake contributing to yield increase of soybean cultivars grown in Mollisols. Plos One, 12(5). doi:10.1371/journal.pone.0176688
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- The Forest Canopy: Structure, Roles & Measurement
- Plant Respiration: Its Importance and Applications
- Forest & Plant Canopy Analysis – Tools…
- Stomatal Conductance: Functions, Measurement, and…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- Irrigating with Saline or Seawater






