January 7, 2021 at 10:38 pm | Updated March 15, 2022 at 11:44 am | 6 min read
To meet the needs of a growing population, society faces the challenge of increasing crop production with reduced resources. This challenge is further complicated by increasingly extreme weather conditions induced by climate change. Even crops such as cassava that are adapted to drought have recorded decreases in yield. In Latin America, a group of scientists set out to find the best cultivars and irrigation regimes that could optimize yield and resource use, focusing on crucial physiological processes in drought responses, such as transpiration and stomatal conductance.
Improving Cassava Cultivars Selection and Irrigation Management
Cassava is an important tropical crop that provides affordable and nutrient-rich food to nearly 500 million people in Latin America, Africa, and Asia. Cassava grows on marginal land with low fertility in regions with less rainfall. However, even these hardy crops’ yields have been falling due to extreme heat and increasing drought caused by climate change.
Water deficiency affects vegetative growth, tuber yield, and quality. Scientists have found that the early tuber filling stages are the most sensitive to drought. In dry conditions, the plant closes stomata to reduce water loss. However, this also results in a reduction of carbon dioxide supply used in the photosynthesis cycle, which affects tuber formation.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Irrigating fields is an ideal solution if provided in the right amounts and targeted at the right times. For example, too much water during the initial stages can increase only vegetative growth but not yield. So, learning how different cultivars respond is crucial.
About 45% of cassava in Latin America grows in dry regions. Hence, a collaborative group of Columbian and Venezuelan scientists, Pacheco, Macias, Campos, A.J Izquierdo, and A. G. Izquierdo, wanted to test the response of local cassava cultivars to irrigation regimes throughout their crop cycle.
Eight clones with potential drought-proofing behaviour were chosen for the experiment: Bolívar 32, Concha Rosada, Guajira, Guajira 1, Guajira 3, Guajira 4, MeVen 77-3, and MeVen 77-1.
In a split-plot experiment, crops were irrigated for the first 15 days to allow establishment. The main treatment was three watering regimes, where crops went for 7, 15, and 21 days without irrigation (DWI). The secondary plots had eight cultivars and four replications. The experiment was conducted between 21 to 111 days after sowing.
The researchers recorded physiological and agronomic variables in the 7th and 17th week.
It was easy to measure the agronomic variables of foliar area, number of leaves, plant height, fresh and dry biomass, and yield.
Recording the physiological processes was more complicated. The degree of greenness was measured by a chlorophyll meter and xylematic hydric potential by using the laboratory method by Scholander et al. (1965).
The scientists were looking for an easy yet accurate means of measuring the gas exchanges in the plants, especially transpiration and stomatal conductance, that remove water from the plant.
Challenge: Finding A Tool to Measure Gas Exchanges
Some of the traditional methods to estimate stomatal conductance are to measure the pore size and density using stomatal imprints taken on a media of cellulose-di-acetate or polymethyl-metacrylate and then analyzing the imprint with a light microscope to count stomata and measure its size.
Another method is dehydration leaf assays. Scientists have to detach leaves, then weigh and place them in a plastic box with a saltwater solution. The whole apparatus is kept in a growth chamber with constant temperature and light intensity. The researchers must weigh the leaves every 30 minutes for three hours. After three hours, dehydration is measured by leaf weight loss.
All these methods are destructive, laborious, and require a laboratory that is well equipped with special equipment and/or chemicals.
There are many methods of measuring transpiration, varying from simple to complicated. The simplest involves cutting a leaf and putting it in a test-tube of water and adding oil to cover the surface of the water. Weighing this at the start and after a certain period of time gives the transpiration rate.
Gravimetric analysis is suitable only for plants in pots and not those growing in the soil, as the whole potted plant must be weighed periodically. The heat pulse velocity method may be more sophisticated but it is destructive; it involves boring two holes reaching 2mm deep into the sapwood and then measuring the transfer of heat from one hole to another.
Solution: The CI-340 Handheld Photosynthesis System
Instead of using difficult and separate methods to measure the three gas exchanges, the scientists decided to use an infrared gas analyzer that could record all three processes.
They found the ideal solution in the CI-340 Handheld Photosynthesis System.
The scientists measured all three processes between 11:00 to 13:00 h. Two leaves in two randomly selected plants per treatment were tested, so a total of 48 leaves were tested in week 7 and another 48 leaves in week 17.
This was a large number of tests to perform, and the scientists’ task was made easy by the simplicity of the CI-340, manufactured by CID Bio-Science Inc. It consists of a leaf chamber, which is directly connected to the carbon dioxide and water vapor gas analyzer.
Transpiration is the loss of water vapor from the plant, which was measured using the CI-340 by recording the levels in the air before it enters and after it leaves the leaf chamber.
The CI-340 measures stomatal conductance, or the openness of stomata, via the rate of transpiration as a function of leaf temperature.The CI-340 has an incorporated non-contact infrared sensor to measure temperatures and didn’t require use of additional tools.
Photosynthesis was measured as the difference in carbon dioxide in the air before and after it left the leaf chamber.
Benefits: Versatile and Accurate Photosynthesis Measurement
The CI-340 is non-destructive; there was no need to harvest leaves, thereby preventing stress and leaf area loss making the tests more accurate.
The device is suitable for field use, as it is light and designed for single-handed operation. Moreover, the CI-340 can withstand air temperatures of 0-450C and relative humidity as high as 95%. As the rechargeable battery gives 4-5 hours of charge, the scientists had ample time to collect data without interruption. A battery charger included in the kit can be used at the end of the day to recharge the instrument.
Scientists were able to choose from ten chambers customized for varying leaf types and shapes to find one suitable for cassava.
Each reading took less than a minute and was stored in the tool. Later, the scientists transferred the data to their computer with the help of an included USB cable.
Stomatal Conductance and Transpiration Reduce Drought Effects in Cassava
Stomatal conductance did not vary between cultivars or irrigation regimes, suggesting that watering at an interval of 21 days was enough to maintain plant health. The scientists think that leaf orientation and canopy architecture could be factors helping the cassava plant.
However, some differences in transpiration were seen. Transpiration was highest in Concha Rosada at 7 DWI and in Guajira at 15 DWI, and recorded 1.83 and 1.75 mmol H2O m-2 s-1, respectively, in comparison to 21 DWI; see Table 1.
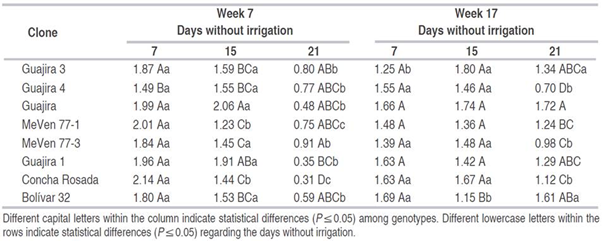
Table 1: “Transpiration (mmol H2O m-2 s-1) measured in weeks 7 and 17 of the evaluation in three irrigation levels and eight cassava clones,” Pacheco et al. 2020. (Credits: http://dx.doi.org/10.15446/rfnam.v73n1.75402)
There were no significant differences in the xylematic water potential between irrigation regimes or cultivars. Nor was there any significant effect of drought on vegetative growth, leaf expansion, or the fresh and dry biomass in any cultivar. However, Guajira 3 and Guajira 1 recorded the best yield parameters.
The cassava performed best when irrigated once in 15 days and had a better gas exchange and vegetative growth.
Overall, the cassava cultivars that were irrigated once in 21 days suffered the most in terms of growth and leaf expansion, and they partially closed their stomata to reduce water vapor loss. However, the impact on photosynthesis and yield in all three treatments, including those irrigated once in 21 days, was not reduced significantly. This proves that the cassava is well adapted to drought.
Based on the study, we can see that irrigation once in 15 days is ideal; however, it can be reduced to once in 21 days to save water without decreasing yield.
Guajira, Guajira 3, Guajira 4, Concha Rosada, and MeVen 77-1 were the best cultivars among the eight tested.
Findings in Latin America
Crop performance can vary between regions due to a combination of soil, environment, and genotype. The scientists were able to prove that cassava cultivars used in Venezuela were drought-adapted and were able to select the best varieties. More importantly, via smart farming, they were able to fine-tune irrigation management so that farmers could produce cassava, a crucial staple food, using less water.
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature image courtesy of Long Lab
Sources
Henslow, G. Simple Methods of Measuring the Transpiration of Plants. Nature 30, 146 (1884). https://doi.org/10.1038/030146a0
Hopper, D., Ghan, R. & Cramer, G. A rapid dehydration leaf assay reveals stomatal response differences in grapevine genotypes. Hortic Res 1, 2 (2014). https://doi.org/10.1038/hortres.2014.2
Meister M.H., Bolhàr Nordenkampf H.R. (2001). Stomata Imprints: A New and Quick Method to Count Stomata and Epidermis Cells. In: Reigosa Roger M.J. (eds) Handbook of Plant Ecophysiology Techniques. Springer, Dordrecht. https://doi.org/10.1007/0-306-48057-3_17
Pacheco, R. I., Macias, M. P., Campos, F. C., Izquierdo, A. J., & Izquierdo, G. A. (2020). Agronomic and physiological evaluation of eight cassava clones under water deficit conditions. Revista Facultad Nacional De Agronomía Medellín, 73(1), 9109-9119. doi:10.15446/rfnam.v73n1.75402
Pegman, A. (2017). Re: Is there any method to measure Stomatal conductance without leaf porometer? Retrieved from: https://www.researchgate.net/post/Is_there_any_method_to_measure_Stomatal_conductance_without_leaf_porometer/5a0f638fed99e154a752b312/citation/download.
Wijeratne, T. (2019). Re: Is there any method to measure Stomatal conductance without leaf porometer? Retrieved from: https://www.researchgate.net/post/Is_there_any_method_to_measure_Stomatal_conductance_without_leaf_porometer/5d6cea644f3a3e463f5215bd/citation/download.
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- The Forest Canopy: Structure, Roles & Measurement
- Plant Respiration: Its Importance and Applications
- Forest & Plant Canopy Analysis – Tools…
- Stomatal Conductance: Functions, Measurement, and…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- Irrigating with Saline or Seawater






