December 5, 2020 at 10:50 pm | Updated December 5, 2020 at 10:50 pm | 8 min read
The symbiosis between the roots of plants and fungus is considered to be widespread and one of the oldest mutualistic associations. One such association is ectomycorrhiza, which is common among tree species. Their role in the growth, health, and productivity of both natural and commercial ecosystems has only recently begun to be appreciated. Studying the role of ectomycorrhiza often involves calculating their biomass as well as their effects on the trees, which can be challenging.
What is Ectomycorrhiza?
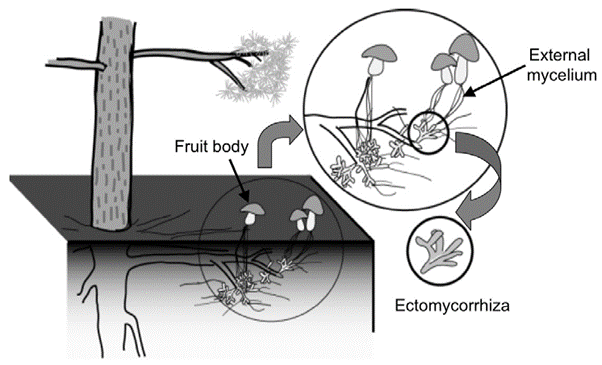
Figure 1: “Schematic diagram of a plant-ectomycorrhizal association,” Satomura et al. 2006. (Image credits: DOI: 10.3117/rootres.15.119)
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Mycorrhizae are formed as a symbiosis of the fungal symbiont or mycobiont with plant roots. About 6% of all plant species rely on this association to meet their nutrient needs. Ectomycorrhiza (ECM) exists mainly as an external coating on the roots, and endo-mycorrhiza enters the root tissue; see Figure 1. Some mycorrhiza can live both externally and internally in roots.
Ectomycorrhiza is associated mainly with woody trees and involves approximately 6,000 plant species and 20,000–25,000 fungal species. These fungal species belong to 162 genera from mainly Basidiomycotina, Ascomycotina, and Zygomycotina.
There are four main parts of the ectomycorrhiza association with trees: the Hartig net, mantle, extraradical hyphae, and fruiting bodies; see Figures 1 and 2.
- Hartig net is the hyphae that penetrate the trees’ roots and exist in contact with the root cells, where the exchange of carbon, nutrients, and water happens.
- Mantle is the hyphal sheath that covers the roots and has more biomass than the Hartig. The mantle can be structured or form a loose net and often suppress the growth of root hairs from the plant symbiont.
- Extraradical hyphae grow outwards from the mantle into the soil and compensate for the loss of root hairs. They can spread over large areas of the soil and can sometimes infect other host plants. When this happens, they allow for the sharing of nutrients between neighboring plants.
- Fruiting bodies are the reproductive parts of the mycobiont and are visible above ground. They have a variety of shapes, such as mushrooms or truffles.
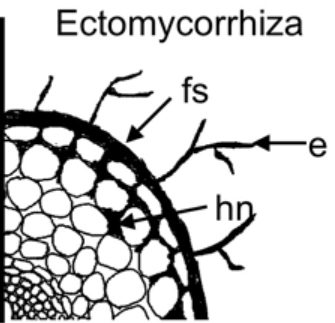
Figure 2: The fungal parts are: “hn, Hartig net, fs, fungal sheath/mantle, and e, external mycelium,” Satomura et al. 2006. (Image credits: DOI: 10.3117/rootres.15.119)
The fungi help in sourcing and transporting nitrogen, which is often deficient. They have been known to provide 86% of the plant nitrogen requirements. The mycobiont also acts as a means of transport for water to roots. Moreover, the mycobiont also protects root tips from pathogens and pests by covering them with hyphae.
In return, plants give carbon in the form of carbohydrates to the mycobionts.
Roles of Ectomycorrhiza
Ectomycorrhiza have several roles in their ecosystems and forestry as a whole. These roles include the following:
Commercial forestry: Trees used in commercial forestry, especially conifers, are inoculated with ectomycorrhiza to encourage growth.
Forest restoration: Disturbance to soil usually destroys the communities of fungi that live in them. So, ecosystem restoration projects include building up fungi populations through inoculum to help native species establish in impoverished soils.
Phytoremediation: Specific species of fungi can break down persistent organic pollutants or accumulate salts. They are also able to grow in soils contaminated with heavy metals. Therefore, they are ideal in phytoremediation.
Climate change mitigation: By improving the health and productivity of trees and forests, ECM builds above-ground carbon sinks. The mycelial networks are also crucial pathways for carbon transport from trees into the surrounding soil. About 25-63% of forest carbon can be found stored in the soils.
Climate change adaptation: As mycobionts help in water sourcing, they can help trees tide droughts which are increasing and becoming more severe due to climate change.
The efficiency of ECM symbiosis is not uniform in all ecosystems, and many human activities—like the addition of fertilizers, destruction of soil structure, and rise in carbon dioxide levels—produce variation in the concentration of fungi. A constant value of 40% was previously used to estimate fungal biomass, but this is unsuitable for several forest types. Hence, individual measurements are necessary.
We will consider two examples to show how and why ectomycorrhiza is estimated to understand its role.
Efficiency of ECM to Alleviate Saline Stress
About 7% of the earth is affected by salinity, and this area is increasing due to irrigation practices and the clearing of land for agriculture. Mycorrhizal interactions are being used to desalinate soils or colonize them by using salt-tolerant species.
Often, tree species become salt-tolerant due to their symbiosis with ECM, but the role of the association is not well understood.
Coccoloba uvifera, or seagrape, is a small tree found growing in coastal regions of the tropics and sub-tropics. They are used as food producers, wind shelters, and as ornamental trees in the Caribbeans.
It is known that the seagrape grows with ECM species of the genera Amanita, Inocybe, Lactarius, Russula, Xerocomus, and Scleroderma. However, the role of ECM in providing nutrition or stress relief was investigated only recently, in 2006, in greenhouses by Bandou et al.
The scientists grew seedlings from seeds for four weeks in tap water without salt. After establishment, the seedlings were subjected to four salt or NaCl treatments (0, 10, 20, and 30%). Fresh salt was added each week, and levels were monitored with a refractometer. Half of the seedlings in each treatment were inoculated by Scleroderma bermudense as the ECM.
The experiment was continued for 12 weeks, after which the seedlings were harvested. The minimal leaf water potential and leaf area were measured.
The CI-203 Handheld Laser Leaf Area Meter produced by CID Bio-Science Inc. was used to record the leaf areas. This is a small, portable device that can also make non-destructive measurements. Each reading takes only a few seconds, and this instrument is ideal for outdoor data collection in greenhouses or the field. Data can be stored and transferred later via wi-fi SD card or USB. The leaf length, width, area, and perimeter are also calculated, as is the Leaf Area Index, using preloaded scientific formulae.
Later, shoots and roots were separated and their collar diameter, height, and dry weight were recorded.
Ten lateral roots of inoculated and non-inoculated plants were washed and examined for ECM growth to estimate colonization through microscopic observation for Hartig net and mantle development. The ECM biomass was also estimated.
The amount of phosphorus (P), potash (K), chlorine (Cl), and sodium (Na) in leaves, shoots, and roots was also estimated.
In inoculated seedlings, S. bermudense produced a white smooth mantle on the roots, but the extent was reduced by higher salinity.
S. bermudense improved the growth of seagrape seedlings regardless of the salinity level.
Height and collar measurements were similar, and salinity reduced the biomass of both ECM plants and those without ECM. However, the leaf, shoot, and root biomass improved in ECM plants and was higher than the controls by 120%.
It was clear that, as the salinity increased, the seagrape increased their dependency on ECM to function.
As salinity increases, plants usually accumulate Na and Cl in leaves and disturb uptake of water. In ECM plants, the mycobiont reduced the absorption of salts and prevented salt stress in seagrape.
Also, the absorption of P by ECM plants continued, as the ECM prevented the binding of P to soils, thus helping them to grow in saline conditions. The extended hyphae of ECM might have also helped in sourcing more P beyond the seagrape roots. There was also more K accumulation in leaves of ECM plants, helping them to tackle osmotic stress due to salinity.
The number of leaves and leaf area was also greater in ECM plants than in controls, even though salinity effects were evident in both treatments. The leaf water status was higher in ECM plants, even though the leaf area was larger.
Thus, the scientists showed that ECM not only helped seagrape growth in normal conditions, but it also increased seagrape tolerance to salinity. Hence, they recommend using an inoculation of ECM to improve the growth and productivity of seagrape.
Estimating ECM in Forests Soils
Given the important role of ectomycorrhiza in maintaining productivity, drought-proofing, and carbon cycling in forests, scientists wanted to find ways of estimating the production, biomass, and turnover of extraradical hyphae, also called extramatrical mycorrhizal mycelia (EMM).
Many older methods do not differentiate between roots and fungi and treat their contribution to carbon cycling as a single organ. However, scientists were interested in finding how much the mycobionts alone contributed.
They chose the temperate and boreal forests, as conifers are the major tree groups that form ectomycorrhiza with fungi.
The scientists reviewed several methods and found that all of them have advantages and disadvantages.
Therefore, they recommended combining two or more methods based the needs of any individual study.
The following methods were reviewed to measure EMM production:
- Minirhizotron observation
- Root free in-growth mesh-bags or cores
- Classification of ectomycorrhizal fungi for indirect measurements
Five methods were used to estimate the biomass of ECM mycelium (EMM):
- Direct measurement of mycelium length in the soil
- Root free in-growth mesh-bags or cores
- Chemical markers in combination with incubation
- Molecular DNA and RNA estimation methods
- Assessment of exploration types
Turnover of ECM mycelium (EMM) was estimated by the following methods:
- Direct minirhizotron
- Direct measurements in growth mesh-bags
- Isotopic techniques
Minirhizotron Observation of ECM Mycelium
Repeated non-destructive estimation is possible with minirhizotrons, so the entire cycle of fungal development can be observed without disturbing them. Minirhizotrons are suitable for estimating production and turnover, as individual hyphae can be followed from production to death. This method requires high-resolution images from modern tools.
The CI-600 In-Situ Root Imager, produced by CID Bio-Science Inc., was one of the two minirhizotrons often used in these direct observations. It is a portable device that can be inserted into installed root tubes, and the scanner can be rotated 360 degrees to take high-resolution images of the roots and hyphae. The accompanying RootSnap! software analyzes the images to calculate length, width, branching, and area of hyphae.
The frequency of data collection and the length of the experiment are crucial for determining the accuracy of this method.
Root Free In-growth Mesh Bags or Cores
Mesh bags or cores are easy and cheap for estimating production. It can also be used in combination with chemical markers, DNA estimation, and isotopically labeled materials for biomass and turnover estimation. Chemical markers are useful as they are highly sensitive to even small amounts of EMM production.
However, relative comparisons may be more efficient than absolute values. This method is destructive, and interactions with soil animals are restricted. Moreover, growth and turnover can be different in bags than in other parts of the soil. The lag time for fungal colonization is long; therefore, results from this method may be unreliable.
Indirect Measurements Through the Classification of ECM
The indirect method relies on information collected through prior laboratory studies to advise on the production levels of different ECM communities. It can be used to estimate production, biomass, and turnover.
However, laboratory rates of growth and production can differ from natural soil conditions. Moreover, only 5-10% of ECM have been studied and classified. Therefore, it is better to combine this technique with molecular methods to estimate biomass.
Molecular DNA and RNA can be used to calculate the biomass of individual species and is suitable for the dominant species. However, this method is expensive, and primers have not been developed for all species.
The scientists recommend testing the methods in different habitats and estimating more mycorrhizal types so that the information collected can shed better light on the biogeochemical cycling of nutrients.
Importance of Ectomycorrhiza Should Increase in Future
Mycobionts form a major component in the soil microbial environment, and their role in the functioning of most ecosystems cannot be overestimated. So, in the future, scientists recommend that ECM contributions should be incorporated into ecosystem models when estimating their productivity and resilience.
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature image courtesy of Kalle Gustafsson
Sources
Bandou, E., Lebailly, F., Muller, F. et al. (2006). The ectomycorrhizal fungus Scleroderma bermudense alleviates salt stress in seagrape (Coccoloba uvifera L.) seedlings. Mycorrhiza 16, 559–565. doi.org/10.1007/s00572-006-0073-6
Peay, Kabir G.; et al. (2007). A strong species–area relationship for eukaryotic soil microbes: island size matters for ectomycorrhizal fungi. Ecology Letters. 10 (6): 470–480. doi:10.1111/j.1461-0248.2007.01035.x. PMID 17498146.
Satomura, T., Hashimoto, Y., Kinoshita, A., & Horikoshi, T. (2006). Methods to study the role of ectomycorrhizal fungi in forest carbon cycling 3: Quantification of the amount of carbon consumed by ectomycorrhizal fungi in a Japanese red pine forest. Root Research, 15(4), 155-159. doi:10.3117/rootres.15.155
Tedersoo, Leho; May, Tom W.; Smith, Matthew E. (2010). Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza. 20 (4): 217–263. doi:10.1007/s00572-009-0274-x. PMID 20191371
Wallander, H., Ekblad, A., Godbold, D., Johnson, D., Bahr, A., Baldrian, P., . . . Rudawska, M. (2013). Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils – A review. Soil Biology and Biochemistry, 57, 1034-1047. doi:10.1016/j.soilbio.2012.08.027
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- The Forest Canopy: Structure, Roles & Measurement
- Plant Respiration: Its Importance and Applications
- Forest & Plant Canopy Analysis – Tools…
- Stomatal Conductance: Functions, Measurement, and…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- Irrigating with Saline or Seawater






