February 20, 2023 at 8:56 pm | Updated February 20, 2023 at 8:56 pm | 8 min read
With the world clamoring to limit climate change, the focus on carbon pools has never been greater. Accurate measurement of carbon pools and tracking changes is crucial for designing and evaluating programs to mitigate global warming or monitor climate change effects. Several approaches exist to measure carbon pools, in which advanced plant instruments are crucial for measurements. But first, it is necessary to understand how to estimate carbon pools.
What are Carbon Pools?
Carbon pools are the places that can accumulate, store, and release carbon. The quantity of carbon in a pool at a specified time is called carbon stock and is measured in gigaton (GtC), where one gigaton equals one billion tonnes.
Carbon fluxes are the processes that move carbon from one pool to another. A carbon pool acts as a sink if the amount of carbon accumulates more than released within a given period. The process by which carbon increases in a pool is called carbon sequestration.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
There are four significant categories of carbon pools:
● Earth’s crust with ~100,000,000 GtC in the sedimentary rocks
● The ocean with ~38,000 GtC
● Terrestrial ecosystems with ~3,000 GtC of organic matter
● The atmosphere with ~750 GtC, whose increasing stock is causing climate change
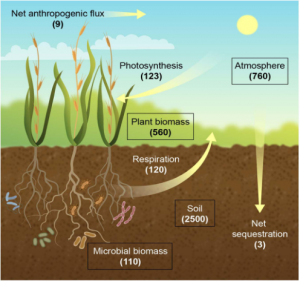
Figure 1: “The terrestrial carbon (C) cycle. Carbon stocks (boxes) are shown as gigatons (GTC), and fluxes (arrows) are shown in GT per year. Respiration refers to accumulated plant and microbial respiration,” Jansson et al. 2021. (Image credits: https://www.frontiersin.org/articles/10.3389/fpls.2021.636709/full)
Terrestrial Carbon Pools
The organic matter that makes up terrestrial carbon pools can be living matter like plants, animals, and microorganisms. Or dead matter like litter, decomposing animals, plants, and microbes in the soil, and harvested wood.
Thus the terrestrial carbon pool has two main components: aboveground and belowground or soil components.
Forests, pastures, wetlands, and croplands are the major terrestrial carbon pools. Forest is the most significant part of the aboveground terrestrial pool, containing 560 GtC. Soils have 2500 GtC, with 1500 GtC of organic carbon and 950 GtC of soil inorganic carbon, and 110 GtC of microbial biomass, see Figure 1.
How Terrestrial Carbon Pools Change
Measuring biomass is a primary means to estimate terrestrial carbon pools, as carbon forms nearly 50% of the biomass.
As Figure 2 shows, there are many pathways by which carbon is added and removed from terrestrial carbon pools.
In the case of vegetation, green plants use carbon dioxide (CO2) from the air and water from the soil in the presence of light to produce simple sugars during photosynthesis, which serves as food for the plants. However, all of this doesn’t form biomass. Instead, plants allocate and partition a large portion of the photosynthates for respiration, growth, and maintenance of the plant structure above and below ground.
Stems and roots are enduring sinks within plants that accumulate carbon and are of interest to climate scientists. Reproductive and propagation parts like flowers, seeds, fruits, and tubers are transient carbon sinks in a plant with commercial value. Their biomass is also crucial for food security.
About 70-80% of carbon allocation goes to aboveground vegetation and 20–30% to belowground tissue. Roots use only half of this carbon for growth. Root respiration and root exudates account for 30% of carbon loss. Mycorrhiza and other microbes use exudates to build their biomass. Decomposition of litter and dead roots, air percolation into the ground, and microbial and root respiration add CO2 to the soil.
Both soil and aboveground parts of vegetation also lose carbon when they release volatile carbon compounds (VOCs) and methane (CH4).
Figure 2. “Transfer of atmospheric CO2 into biotic and pedologic carbon (C) pools of the plant ecosystem. Carbon is lost from the ecosystem as CO2, volatile organic compounds (VOCs), and methane (CH4),” Jansson et al. 2021. (Image credits: https://www.frontiersin.org/articles/10.3389/fpls.2021.636709/full)
Allometric Models
Biomass measurements are possible at the ecosystem or the plant level since plants are the primary producers in terrestrial ecosystems.
Methods to measure changes in carbon pool can be direct or indirect, destructive or non-destructive.
Direct destructive methods involve cutting down parts of trees like stems, foliage, branches, or even the entire plant to measure the biomass. Direct methods are accurate but time-consuming, labor-intensive, and difficult.
However, it is possible to use allometric models based on the correlation between morphological and physiological characteristics and biomass. The physiological features are those directly involved in carbon fluxes, like respiration and photosynthesis, or those that influence them, like chlorophyll content, stomatal conductance, and transpiration. Morphological features are sink or source parameters, such as plant height, basal tree diameter, canopy cover, and leaf area index.
When combined with remotely sensed satellite imagery, these allometric methods can estimate carbon at the field level or even larger scales. In the following section, we discuss the methods used for plant-level estimations.
Photosynthesis
Scientists have established that terrestrial ecosystems sequester 123 GtC of CO2 each year from the atmosphere through photosynthesis. In addition, many commercially available portable tools make non-destructive measurements in the field based on different aspects of photosynthesis.
Tools like Infrared Gas Analyzer measure the amount of CO2 plants use, as the difference in CO2 levels in the incoming and outgoing air. This is because CO2 absorbs infrared wavelengths of light, and when leaves use CO2, there is less gas in the outgoing air and, therefore, less infrared light absorption.
Alternatively, you can also measure O2 produced during photosynthesis with electrochemical sensors. For example, CID Bio-Science’s CI-340 Handheld Photosynthesis System uses both these methods for gas exchange measurements.
Chlorophyll fluorescence measurements are another way to measure photosynthetic rates and CO2 assimilation.
Respiration
Plants use 40-60% of the carbon fixed during photosynthesis for respiration. Green plants respire aerobically and burn the photosynthates using O2 to produce energy and CO2. Carbon skeletons, byproducts of carbon metabolism in the Citric Acid Cycle, form the basis of all plant bio compounds.
It is not only leaves, but also roots that respire. Root respiration accounts for 50% of soil respiration, a significant soil carbon flux.
Gas exchange measurements are the standard method to estimate aerial respiration, which is possible with the CI-340 Handheld Photosynthesis System, manufactured by CID-BioScience Inc. The tool can measure both CO2 and O2 levels accurately in the field.
Transpiration and Stomatal Conductance
Transpiration influences photosynthesis in many ways and is crucial for carbon fixation.
Loss of water vapor during transpiration occurs mainly through the stomata in leaves. Stomata are the openings formed when the guard cells open when they are turgid due to high cell moisture content. CO2 needed for photosynthesis and O2 for respiration can only enter when stomata are open. So, transpiration and stomatal conductance control both carbon assimilation and metabolism.
Depending on the location of the stomata and the timing of stomatal conductance, there are three photosynthetic pathways.
Transpiration also pulls nutrients from the soil, including water, necessary for photosynthesis. The CI-340 Handheld Photosynthesis System can simultaneously measure transpiration and stomatal conductance with photosynthesis, providing scientists with an overall view of associated conditions.
Plant Size
Plant allometry is the measurement of the size or growth of the entire plant or even parts. Plant parts used for biomass estimations in aboveground vegetation are height, basal area, and foliage.
Various methods exist to measure plant height depending on the size. For smaller herbs and shrubs, a simple measuring tape is enough. For tall trees, standard methods are the tangent and sine methods with laser rangefinders. The tangent method uses the horizontal distance to the tree and its angles to the base and top of the tree.
The sine method measures the distance to the base and top of the tree and the angles made by the horizontal with both these distances.
The basal area is the cross-sectional area of a stem/stems at breast height. Allometric equations for individual trees are scaled up to produce stand-level models by including plot density and considering the relationship between density and average tree size.
Foliage
The amount of foliage a plant reflects on its carbon-fixing capacity. In biomass estimations, foliage contributions to carbon measurements are possible at cellular, individual, and plot levels.
Leaf Chlorophyll
The amount of chlorophyll in the leaves indicates productivity and carbon fixation efficiency at the cellular level, as it is vital to light use during photosynthesis. Spectroscopy, field tools, or remote satellite imagery, pigments are easily estimated. Field tools such as the CID’s CI-710s SpectraVue Leaf Spectrometer are useful for cropland, forests, and wetlands. The device has preloaded vegetative indices suitable for a variety of studies.
Leaf Area Index (LAI)
LAI is the leaf area per unit ground area. It measures foliage at the individual plant level and is used widely for croplands and natural ecosystems. LAI provides information on the surface available for radiation and rainwater interception, photosynthesis, and transpirational losses.
LAI measurements are widespread, and there are several methods-direct and indirect. Many methods are destructive. However, commercial tools for non-destructive estimations also exist:
- The portable CI-110 Plant Canopy Analyzer uses a 150-degree hemispherical lens to make images from beneath the canopy to calculate LAI.
- LAI estimations are also possible by leaf area measurements with the help of non-destructive handheld CI-203 laser leaf Meter and CI-202 Portable Laser Leaf Area Meter.
Canopy cover
Canopy Cover is the arrangement of crowns of many individuals and species and is measured at several large scales, from local to regional. Several existing models consider the vertical and horizontal components of canopy cover when measuring carbon pools and their changes.
Remote sensing is the standard measurement method for estimating large-scale canopy cover. At the plot level, instruments for canopy cover measurements include the convex spherical densiometer, angular densiometer, moosehorn, canopy scope, vertical tube, handheld sensors, and Hemispherical photography.
The portable CI-110 Plant Canopy Analyzer , based on Hemispherical photography, is designed to estimate canopy cover with the Gap Fraction method.
Soil Carbon
Even though soil carbon accounts for 80% of the terrestrial carbon pool, its importance has been recognized only recently. Soil organic matter is composed of microbial biomass and decomposing plant and animal tissue. The plant tissue can be litter from aboveground parts and roots. Moreover, carbon pool measurements must also include live roots. Root-derived carbon is 2.4 times more likely than shoot-derived carbon to stabilize and add to enduring soil carbon.
Due to difficulties in measuring root growth, roots’ contributions to soil carbon have been long neglected. However, new minirhizotrons such as CID’s CI-600 In-Situ Root Imager and CI-602 Narrow Gauge Root Imager allow scientists to take high-resolution non-destructive images to accurately estimate root length, diameter, area, and volume. In addition, studies have identified live and dead roots to quantify growth and root biomass based on appearance. Measurements are also possible over multiple seasons.
Soil organic carbon can be inferred, at least partially, by indirect methods based on carbon fluxes through underground plant activities and changes in aboveground vegetation.
At large regional scales, multispectral and hyperspectral bands successfully quantify soil organic carbon with models. Other methods include direct carbon estimation in the soil through laboratory analysis, spectral measurements, and soil bulk density.
A Mix of Old and New
The above list of methods to measure carbon pools only covers plant-related measurements. Many approaches to measuring natural ecosystems’ biomass and croplands are new applications of decades-old techniques. Similarly, older vegetative indices and models to analyze vegetation are being adapted or improved with further information from fields and remote sensing to cover large areas and answer specific questions related to carbon storage. In addition, the tools used for precise real-time field measurements are new, offer an alternative to tedious laboratory wet methods, and help speed up research and practice of carbon pool measurement.
Sources
Engku Ariff, E. A. R., Suratman, M. N., & Abdullah, S. (2015). Allometric Equations for Estimating the Carbon Sequestration in Rubber Plantations. Journal of Tropical Resources and Sustainable Science (JTRSS), 3(3), 51–60. https://doi.org/10.47253/jtrss.v3i3.537
Jansson, C., Faiola, C., Wingler, A., Zhu, X.-G., Kravchenko, A., de Graaff, M.-A., Ogden, A. J., Handakumbura, P. P., Werner, C., & Beckles, D. M. (2021). Crops for carbon farming. Frontiers in Plant Science, 12. https://doi.org/10.3389/fpls.2021.636709
Han, S. H., & Park, B. B. (2020). Comparison of allometric equation and destructive measurement of carbon storage of naturally regenerated understory in a pinus rigida plantation in South Korea. Forests, 11(4), 425. https://doi.org/10.3390/f11040425
Hanania, J., Stenhouse, K., & Donev, J. (2020). Energy Education – Carbon pool [Online]. Available: https://energyeducation.ca/encyclopedia/Carbon_pool. [Accessed: April 12, 2022].
Hofstad O: Review of biomass and volume functions for individual trees and shrubs in Southeast Africa. J Trop for Sci 2005, 17: 151–162.
Larjavaara, M., & Muller-Landau, H. C. (2013). Measuring tree height: A quantitative comparison of two common field methods in a moist tropical forest. Methods in Ecology and Evolution, 4(9), 793–801. https://doi.org/10.1111/2041-210x.12071
Ontl, T. A. & Schulte, L. A. (2012) Soil Carbon Storage. Nature Education Knowledge 3(10):35. Retrieved from https://www.nature.com/scitable/knowledge/library/soil-carbon-storage-84223790/
Petrokofsky, G., Kanamaru, H., Achard, F. et al. (2012). Comparison of methods for measuring and assessing carbon stocks and carbon stock changes in terrestrial carbon pools. How do the accuracy and precision of current methods compare? A systematic review protocol. Environ Evid 1, 6. https://doi.org/10.1186/2047-2382-1-6
Rasse, D. P., Rumpel, C., & Dignac, M.-F. (2005). Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant and Soil, 269(1/2), 341–356. http://www.jstor.org/stable/24124940
Smith, P., Soussana, J. F., Angers, D., et al. (2019). How to measure, report and verify soil carbon change to realize the potential of soil carbon sequestration for atmospheric greenhouse gas removal. Global Change Biology, 26(1), 219–241. https://doi.org/10.1111/gcb.14815
Tian, J., Wang, L., Li, X., et al. (2019). Canopy Height Layering Biomass Estimation Model (CHL-BEM) with Full-Waveform LiDAR. Remote Sens.,11, 1446. https://doi.org/10.3390/rs11121446
Torres, A.B., & Lovett, J.C. (2013). Using basal area to estimate aboveground carbon stocks in forests: La Primavera Biosphere’s Reserve, Mexico, Forestry: An International Journal of Forest Research, 86 (2), 267–281, https://doi.org/10.1093/forestry/cps084
The University of New Hampshire. (n.d.). Pools, Fluxes, and a Word About Units. Retrieved from http://globecarboncycle.unh.edu/CarbonPoolsFluxes.shtml
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- The Forest Canopy: Structure, Roles & Measurement
- Plant Respiration: Its Importance and Applications
- Forest & Plant Canopy Analysis – Tools…
- Stomatal Conductance: Functions, Measurement, and…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- Irrigating with Saline or Seawater






