October 21, 2020 at 9:43 pm | Updated March 16, 2022 at 1:27 pm | 7 min read
Irrigation is essential to increase food production. However, competing needs and increasing drought makes water for irrigation scarce. Therefore, new methods and strategies are continuously being tested by scientists to improve food production, accompanied by better water use efficiency. This article will examine how modern field tools are helping in this venture.
Irrigation Methods
According to FAO, although only twenty percent of the land is irrigated, it provides forty percent of the food. With the population set to increase, more land will be added to agriculture, and irrigated farms will expand by thirty-four percent by 2030. However, water is scarce, and only fourteen percent more water can be provided for irrigation.
Of the water drawn for irrigation from groundwater, rivers, or lakes, only forty percent is actually utilized by the plants. The remaining sixty percent of water is lost due to evapotranspiration, infiltration into the soil, or consumption by weeds.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
The choice of the watering method depends on the soil type, topography, climate, water availability, and crops. The main systems used are surface, sprinkler, and drip irrigation. While the latter two are more water-efficient, some plants need flooding, like rice.
Recent studies are being helped by a swathe of new modern instruments that bring sophisticated and complex analysis technology to the field. They provide accurate readings with little investment of time.
Here, we explore some research into novel irrigation ideas.
Water Distribution by Sub-surface Drip Influences Root Distribution
Drip irrigation uses the least amount of water, so new methods in this technique are being developed, including sub-surface drip systems.
In sub-surface drip, the tubes are buried around twelve centimeters below the soil surface to avoid evaporation loss and runoff and to supply water close to the root zone area (see Figure 1). This method reduces weeds and there is less damage to the system by rodents.

Figure 1: The drip tape & its wetting pattern, University of California, Agriculture and Natural Resources. (Image credits: https://ucanr.edu/sites/adi/)
The disadvantage of this system is that roots need to have a flexible root system that can grow to reach localized wet soils.
However, root parameters in drip irrigation have not been studied as often as canopy attributes because they are under the ground and difficult to observe. Information on the root growth in various crops is just beginning to emerge.
New minirhizotrons that can take non-destructive images of roots up to a depth of one meter have provided a breakthrough. Older methods involved destructive and laborious methods like digging to get a soil profile. Therefore, studies that trace the growth of roots of the same plant in response to irrigation throughout the crop cycle are even rarer.
A study in Brazil traces the effects of sub-surface drip irrigation on the root system in three varieties of sugarcane, a perennial plant. Part of the nutrients was supplied by the drip system, and the rest was added as solid fertilizers in the first year after the harvest/ratoon. The water management was aided by a soil water probe. The experiment was conducted between the second and third year of growth.
Scientists used the minirhizotron CI-600 In-Situ Root Imager produced by CID Inc. Biosciences. Transparent tubes are installed by boring holes in the ground. The root imager that has a camera is then inserted into the holes to take scans of the roots growing around the tubes. The accompanying RootSnap software calculated several parameters like root branching, length, diameter, area, and volume. This offered the advantage of observing the changes in roots through the different phases. Moreover, root growth was measured at every twenty-centimeter interval.
Scientists scanned the roots, five times between 38 to 205 days after first ratoon (DAR). They measured the root length with the imager and calculated density for each plot. Soil water was estimated by samples collected from different depths in the ground.
Most of the water, as well as the nutrients phosphorus and calcium added through the drip, was present at a depth of 20 centimeters, where the pipes were laid. This was also the depth where the highest densities of roots were seen, as seen in Figure 2. About eighty percent of the root system was found within a depth of forty centimeters below the soil surface. The scientists found that the maximum rate of root growth was during the earlier stages of the experiment during tiller development. All the three varieties increased their root density up to 205 days of the second ratoon. One of the varieties (a) had a more efficient root system than the other two for the given conditions.
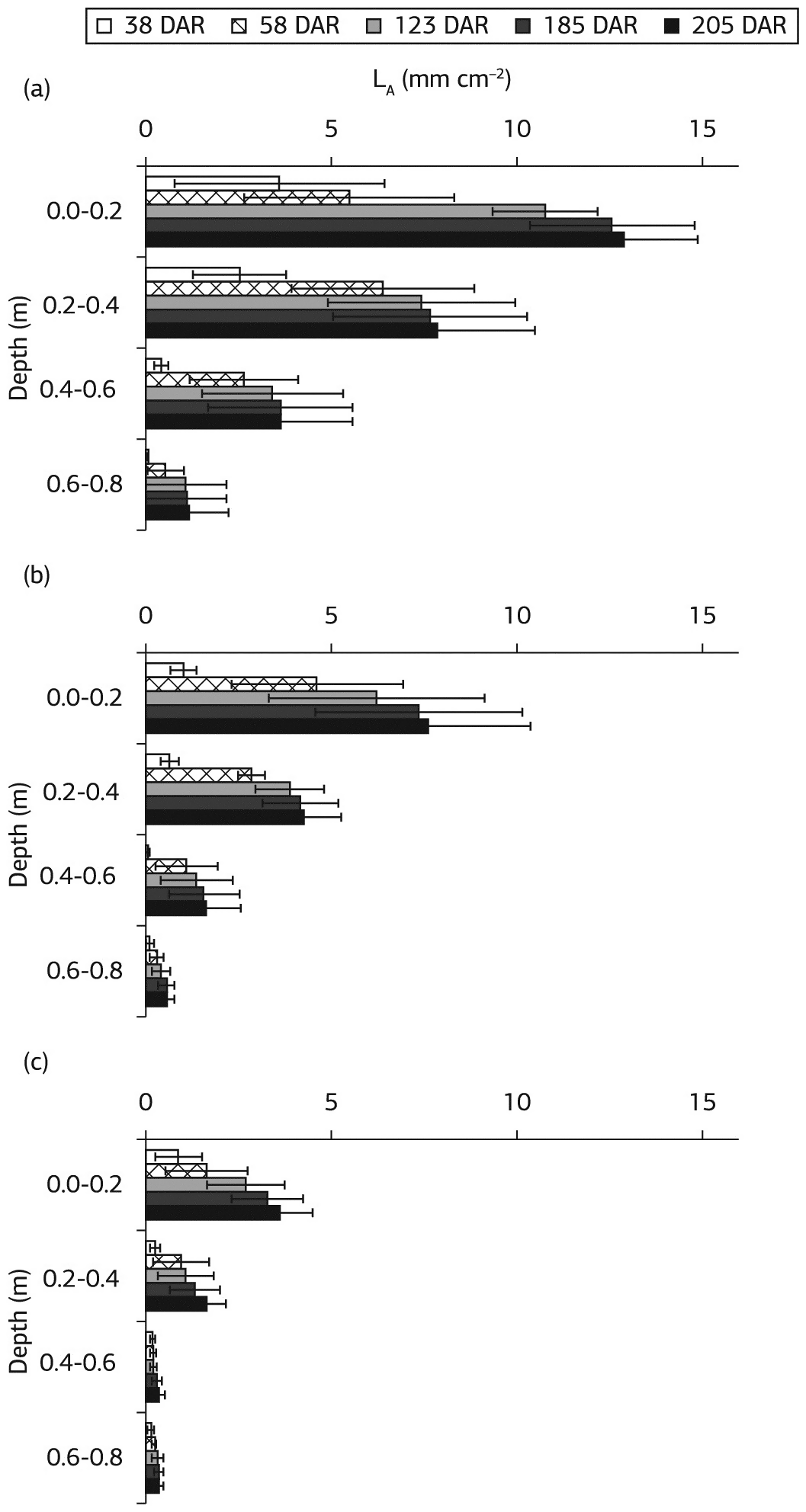
Figure 2: Total root density in three sugarcane cultivars (a), (b), and (c) between 38 to 205 DAR (days after ratoon), Ohashi et al. 2015. (Image credits: http://dx.doi.org/10.1590/1678-4499.0295)
Nutrient Mobility due to Sub-surface Drip
The distribution of water can also influence other processes in the soil relevant to crop growth.
Scientists wanted to test if localized water distribution in drips affected the mineralization of compost or cover crops in organic farms at the Russell Ranch Century Experiment at UC Davis. They had earlier noticed that nutrient mineralization and movement in the soil was different in organic farms compared to conventional plots.
To solve the problem of small wetting by drips, a new system of laying the pipes was tested. Two lines of sub-surface drip for each row of the crop were tested against one sub-surface row of drip and furrow irrigation. Tomato and corn were grown in plots where winter cover crops of beans, oat, and vetch were grown.
The parameters examined were the yield, weeds, soil water, and nitrate levels. They also took scans of the root growth once in a fortnight with the CI-600 In-Situ Root Imager. The accompanying software calculated the length, diameter, area, volume, and branching angle of roots.
The scientists at UC Davis found that tomato and corn roots showed more proliferation in drip than furrow irrigation. As they suspected, nitrates were spread further and the distribution was even by furrow watering. In plots with drip irrigation, nitrates remained closer to the place of organic matter application. Therefore, manure should be placed in the proximity of roots in sub-surface drip systems.
There was no difference in yield between two lines of drip and one line of drip.
Reducing Water Use in Rice
In Asia, half of the water for irrigation is used for growing rice, the staple food of the region. Currently, two to five thousand liters of water are used to produce one kilo of rice. Scientists wanted to develop more efficient methods of irrigation that would also be easy for farmers to adopt.
Scientists conducted an experiment with pots, where rice was subject to the following four treatments:
- T1 was the control. Pots were flooded by five centimeters of water and irrigated when levels dropped to three centimeters.
- T2 pots were flooded at three centimeters and irrigated at levels of one centimeter of water.
- T3 pots were flooded by only one centimeter and irrigated when soil water fell to saturated levels.
- T4 was subject to alternatively dry and wet conditions (flooding of 5 centimeters).
Physiological parameters like chlorophyll content, chlorophyll fluorescence, photosynthetic and transpiration rates, photosynthetic active radiation, and stomatal conductance were measured. Soil samples were analyzed for the major nutrients, pH, and electrical conductivity. These were compared to yield and its parameters from the four treatments.
The CI-340 Handheld Photosynthesis Meter manufactured by CID Inc Biosciences was used to determine photosynthesis (Pn), photosynthetically active radiation (PAR), and stomatal conductance (SC).
The CI-340 is a light and portable device that can be used for non-destructive measurements in the field. It has modules to control temperature, light, water, and carbon dioxide. CI-340 is suitable for open and closed systems to give rapid results. The leaf chambers come in ten sizes for use with leaves of different sizes.
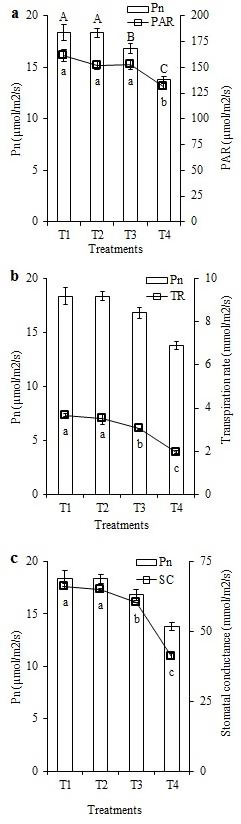
Figure 3: “Effect of different water levels on photosynthetic active radiation (PAR), transpiration rate (TR), and stomatal conductance (SC) were presented as compared with the photosynthetic rate (Pn) in a, b, c [Pn,” Khairi et al. 2015. (Image credits: http://www.cropj.com/jahan_9_2_2015_153_159.pdf)
The number of tillers, grains per tiller, and yield were similar in the first three watering regimes and were significantly less only in the alternate dry and wet treatment. This reflected trends in water-use efficiency and nutrient availability. Levels of chlorophyll content and fluorescence also showed the same pattern.
Photosynthesis, transpiration, and stomatal conductance dwindled with decreasing water and were less in T3 compared to the first two treatments and was the least in the alternate dry and wet treatment.
T3 used forty-five percent less water but gave similar yields obtained from current practices (control T1). It is just necessary to ensure that the soil doesn’t dry up.
Limiting and changing the irrigation method can save considerable quantities of water and give rice production a boost.
Meeting Natural Challenges
Pragmatic and straightforward solutions that can be quickly adopted can optimize farming. New portable instruments, such as the minirhizotron or photosynthesis system, are useful in the field and greenhouses. They offer scientists the possibility of exploring plant processes in depth to fine-tune existing systems of irrigation, giving substantial improvements in food production.
—
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Featured image courtesy of _paVan_
Sources
Caitlin A. Peterson, C.A., Soares, T., Torbert, E., Herrera, I., Scow, K.M., & Gaudin, A.C.M. (2016). Drip Irrigation Effect on Soil Function, Root Systems and Productivity in Organic Tomato and Corn. Proceedings of the Organic Agriculture Research Symposium, Pacific Grove, CA. Retrieved from https://eorganic.info/sites/eorganic.info/files/u27/4.11-Petersonal-Drip_Irrigation_TomatoesCorn-Final.pdf
FAO. Water. Retrieved from http://www.fao.org/water/en/
FAO. Chapter 3. The use of water in agriculture. Retrieved from http://www.fao.org/3/Y4683E/y4683e07.htm
FAO. Chapter 7. Choosing an irrigation method. Retrieved from http://www.fao.org/3/S8684E/s8684e08.htm
Khairi, M., Nozulaidi, M., Afifah, A., Jahan, M. (2015). Effect of various water regimes on rice production in lowland irrigation. Australian Journal of Crop Science, 9:153-159. Retrieved from http://www.cropj.com/jahan_9_2_2015_153_159.pdf
Ohashi, A.Y.P., Pires, R.C.M., Ribeiro, R.V., & Silva, A.L.B.O. (2015). Root growth and distribution in sugarcane cultivars fertigated by a subsurface drip system. Bragantia, 74(2), 131-138. Epub April 29, 2015.DOI: https://dx.doi.org/10.1590/1678-4499.0295
Qiao, X. ( 2018, October 17). Subsurface drip irrigation tested at Panhandle Center in Nebraska. The Fence Post. Retrieved from https://www.thefencepost.com/news/subsurface-drip-irrigation-tested-at-panhandle-center-in-nebraska/.
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- The Forest Canopy: Structure, Roles & Measurement
- Plant Respiration: Its Importance and Applications
- Forest & Plant Canopy Analysis – Tools…
- Stomatal Conductance: Functions, Measurement, and…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- Irrigating with Saline or Seawater






