October 21, 2020 at 9:35 pm | Updated March 15, 2022 at 12:16 pm | 7 min read
Plants face many environmental stresses, such as light, moisture, nutrients, temperature, and pollutants. Many of these factors are essential for plant growth, in optimum quantities; however, a dearth or excess can cause stress. As part of the plant’s overall response to stress, photosynthesis, which is one of the most important metabolic activities, also gets affected. This impact offers the opportunity to monitor stress, as there are highly sophisticated precision tools that can non-destructively measure photosynthesis in the field.
Factors Affecting Photosynthesis
Photosynthesis, one of the important and ubiquitous physiological processes of the plant, doesn’t occur in a vacuum. It is interlinked and affected by other metabolic activities in the plants, including the following:
- Supply of raw materials: Photosynthesis needs raw materials for the reaction, like gases and water. If the supply in leaves is not optimum, the rate of photosynthesis is affected.
- Production of chlorophyll: Chlorophyll, the green pigment where photosynthesis occurs, has to be produced by the plant using minerals and is subject to control by many genes and enzymes. When any of the essential chemicals necessary for the process are not present, the photosynthetic rate can be affected.
- The efficiency of stress coping mechanism: Many compounds are accumulated by plants, especially in leaves, to cope with stress, whether due to heat, drought, or pest injury. These can impact the whole photosynthetic apparatus.
Since photosynthesis is sensitive to alterations in supply, chemical reactions, and other chemicals, changes in its rate can be traced back to the relevant cause to measure several kinds of stress.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Precision Tools for Photosynthesis
The most common stresses are heat and drought, pollutant toxicity, and nutrient deficiency. We will show how photosynthesis, measured using the CI-340 Handheld Photosynthesis System, can estimate these stresses.
The CI-340 Handheld Photosynthesis System, manufactured by CID Bio-Science Inc., is one of the few tools available on the market for this purpose. It is a handy portable tool that uses infrared gas analyzers to measure gas exchange, such as photosynthesis, stomatal conductance, transpiration, and respiration.
The measurements are non-destructive and rapid. It has ten sizes in leaf chambers to fit all species and can be used as an open or closed system. It has customized modules to monitor gases, chlorophyll fluorescence, light, and temperature.
Drought Changes Photosynthesis and Chlorophyll Fluorescence
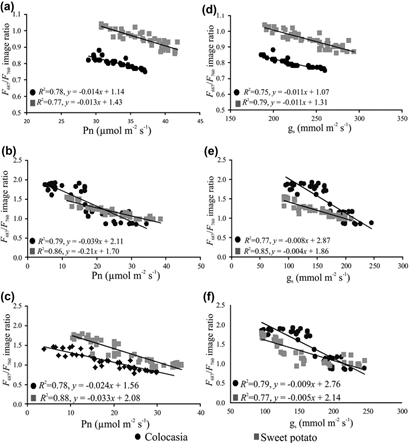
Figure 1: “Relationships between F 687/F 760 ratios with (a) net photosynthetic rate (Pn) for control plants, (b) Pn for herbicide treated plants, (c) Pn for drought-stressed plants, (d) stomatal conductance (gs) for control plants, (e) gs for herbicide treated plants and (f) gs for drought-stressed plants,” Raji et al. 2017. (Image credits: https://doi.org/10.1007/s12524-016-0612-3)
When photosynthesis decreases, less light is needed by chlorophyll in leaves to fix carbon dioxide. The unused light is either dissipated as heat or as fluorescence (F). There are two steps in which light is used: photosystems I (PSI) and photosystems II (PSII). PSI emits fluorescence as far-red light that has a wavelength of 760 nm (F760) and PSII emits it as red fluorescence, which has a wavelength of 687 nm (F687).
PSI is not affected by drought stress, but PSII is. So, when PSII doesn’t function properly, less light is used and the red fluorescence (F687) should increase. The ratio of the fluorescence from the two photosystems F687/F 760 is used to detect drought stress. During drought stress, the value of the ratio will increase.
The collection of chlorophyll fluorescence from sunlight is also called sun-induced fluorescence. It is measured by remote sensing by passive means either from satellites or by proximal remote sensing.
The proximal remote sensing method can make non-destructive measurements of drought in the fields. One such instance was an Indian study, where scientists checked the effect of drought in field-grown colocasia and sweet potato plants using reflectance data from a multi-spectral imaging system.
Meaningful analysis of the chlorophyll fluorescence data is best done in combination with information about the rate of photosynthesis and stomatal conductance to get an overview of associated processes.
The scientists used the CI-340 Handheld Photosynthesis System to measure photosynthesis and stomatal conductance by infrared gas analyzers.
Imaging data were collected from control and treated plants for 14 days. As expected, the drought-affected plants had a higher F687/F760 ratio. For example, the ratio was between 0.75-0.85 for control plants of colocasia, while drought-affected plants registered ratios up to 1.5. Photosynthesis is between 25-45 µmol/m2/s2 in control plants and can drop close to 0µmol/m2/s2 in drought-affected colocasia and never rises above 35 µmol/m2/s2; see Figure 1.
Similarly, in sweet potato, the rate of photosynthesis falls from 30-42 µmol/m2/s2 in control plants to 10-35 µmol/m2/s2 in drought-affected plants; see Figure 1. The F687/F760 ratio was also significantly higher in drought-affected plants and was between 1-2.
The scientists were able to prove that proximal remote sensing of F is a reliable method to detect drought effects in crops.
Phytocystatin Mitigates Cadmium Stress
Phytocastins are crucial in shaping a plant’s response and its ability to tolerate stress. A family of proteins, phytocastins are one of the most important chemicals produced during any stress. The production of phytocystatins increases during heat and injury stress, and they act by inhibiting cysteine proteases.
Cadmium is a toxic heavy metal that is a pollutant often present in agricultural soils and is responsible for reducing crop yields.
A study of two mustard (Brassica juncea L.) cultivars in India showed how phytocastins can influence photosynthesis in the event of cadmium stress.
Ro Agro 4001 and Amruta were the two varieties subjected to 50 µM of cadmium. Photosynthetic rate, chlorophyll fluorescence, fresh mass, and dry mass of the plants were some of the parameters that were recorded. The study also traced nitrogen use and content of cysteine and glutathione in the plants.
Amruta had more cadmium levels in it than Ro Agro 4001; cadmium impacted photosynthesis and decreased plant growth in both cultivars, but Amruta suffered more.
Cadmium acted by reducing chlorophyll content and affected the activity of Rubisco, a part of photosynthesis, and the exchange of gases in both cultivars.
Ro Agro 4001 had higher phytocystatin activity in response to cadmium stress, which helped mitigate the heavy metal’s toxicity. Consequently, this cultivar had higher photosynthetic rates and less fluorescence than Amruta. Ro Agro 4001 also showed better nitrogen use and sulfur assimilation. It also had more cysteine, once again showing that it was protected by phytocystatin.
Consequently, the mustard variety Ro Agro 4001 showed better growth reflected in fresh and dry matter weights.
Zinc Deficiency and Toxicity Impacts Photosynthesis
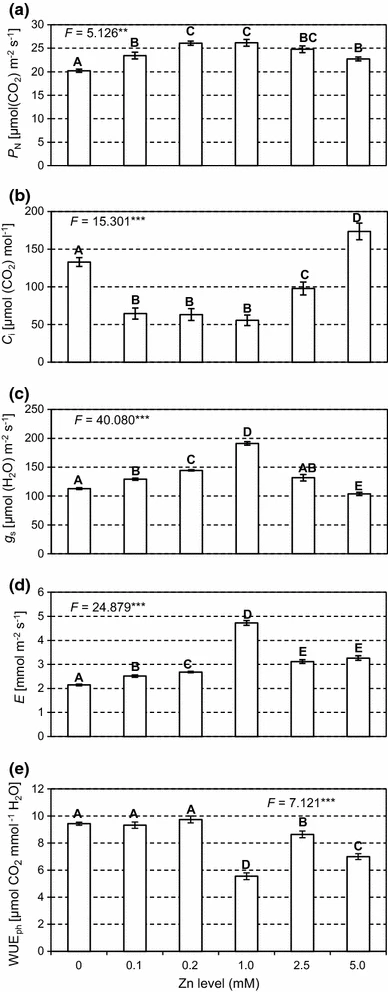
Figure 2: “P N net photosynthesis rate (a), C i intercellular CO2 concentration (b), g s stomatal conductance (c), E transpiration rate (d) and WUEph water use efficiency (e) in Salix leaves at different Zn application levels,” Borowiak et al 2017. (Image credits: Acta Physiol Plant 37, 155. https://doi.org/10.1007/s11738-015-1904-x)
Zinc (Zn) is a micro-nutrient necessary for protein formation and activation of enzymes in plants. The proteins are used in photosynthesis, auxin and nucleic acid synthesis, and carbohydrate, nitrogen, and lipid metabolism. At optimum concentrations, zinc can boost growth, plant productivity, and fruit development.
However, if it is present in higher concentrations, it is toxic to the plant and produces symptoms similar to heavy metal toxicity. It can produce imbalances in photosynthesis, transpiration, synthesis of chlorophyll, and cell membrane integrity.
Both zinc deficiency and toxicity produces chlorosis in leaves due to less chlorophyll and carotenoids.
In a Polish experiment, scientists tested the levels of zinc that willow hybrids could stand, as this species is commonly used for phytoremediation of polluted soils. They used the hybrid Salix purpurea × triandra × viminalis 2.
Salix cuttings were made and grown for twenty-one days in pots subject to five concentrations of zinc: 0.1, 0.2, 1.0, 2.5, and 5.0 mM of Zn.
The CI-340 measured net photosynthesis, stomatal conductance, intercellular CO2 concentration, and transpiration rate.
Levels of chlorophyll, carotenoids, phenols, and carbohydrates in leaves were also estimated. The number and length of leaves and leaf area were measured after twenty-one days.
As expected, the scientists found that at optimum zinc levels of 1–2.5 mM, the plant performance was the best in terms of all the physiological activities measured; see Figure 2.
When zinc levels were below or above this range, there was a decrease in chlorophyll content. Therefore, the photosynthetic rate and amounts of carbohydrates produced were also lower.
However, photosynthesis reduction at toxic levels of 5.0 mM of Zn were lower than when no zinc was given to the plants in control replicates. So, it is possible to grow willows in soil polluted with more than 5.0 mM of Zn.
Phenols are a plant’s response to heavy metal stress. Their concentrations increased as zinc concentrations rose, proving they were a detox method used by the plants.
Making Crop Breeding More Efficient
Crop research is delving deeper into the internal processes of plants to fine-tune yield so that the new cultivars can function not only during optimal conditions but also during stress. By using simple portable tools like the CI-340, scientists and agronomists can analyze photosynthesis to see how various kinds of stress affects plant growth and productivity. It is useful in choosing cultivars that can cope better with stress and help in increasing food security.
—
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature image courtesy of EpicTop10.com
Sources
Borowiak, K., Gąsecka, M., Mleczek, M. et al. (2015). Photosynthetic activity in relation to chlorophylls, carbohydrates, phenolics and growth of a hybrid Salix purpurea × triandra × viminalis 2 at various Zn concentrations. Acta Physiol Plant 37, 155. https://doi.org/10.1007/s11738-015-1904-x
Clevers, J. (n.d.). MSc thesis subject: Sun-induced fluorescence of agricultural crops. Wageningen University & Research. https://www.wur.nl/en/article/MSc-thesis-subject-Sun-induced-fluorescence-of-agricultural-crops.htm
Hwang, J.E., Hong, J.K., Lim, C.J., Chen, H., Je, J., Yang, K.A., Kim, D.Y., Choi, Y.J., Lee, S.Y. and Lim, C.O. (2010) Distinct expression patterns of two Arabidopsis phytocystatin genes, AtCYS1 and AtCYS2, during development and abiotic stresses. Plant Cell Rep. 29, 905–915.doi: 10.1007/s00299-010-0876-y
Per, T.S., Khan, S., Asgher, M., et al. (2016). Photosynthetic and growth responses of two mustard cultivars differing in phytocystatin activity under cadmium stress. Photosynthetica 54, 491–50. doi: https://doi.org/10.1007/s11099-016-0205-y
Raji, S.N., Aparna, G.N., Mohanan, C.N., et al. (2017). Proximal Remote Sensing of Herbicide and Drought Stress in Field Grown Colocasia and Sweet Potato Plants by Sunlight-Induced Chlorophyll Fluorescence Imaging. J Indian Soc Remote Sens 45, 463–475. doi:https://doi.org/10.1007/s12524-016-0612-3
Woolhouse H.W. (1983) The Effects of Stress on Photosynthesis. In: Marcelle R., Clijsters H., van Poucke M. (eds) Effects of Stress on Photosynthesis. Advances in Agricultural Biotechnology, 3. Springer, Dordrecht. doi: https://doi.org/10.1007/978-94-009-6813-4_1
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- Forest & Plant Canopy Analysis – Tools…
- The Forest Canopy: Structure, Roles & Measurement
- The Importance of Leaf Area Index (LAI) in…
- Root Respiration: Importance and Applications
- Stomatal Conductance: Functions, Measurement, and…
- Irrigating with Saline or Seawater
- Plant Respiration: Its Importance and Applications




