October 21, 2020 at 9:39 pm | Updated March 16, 2022 at 11:29 am | 8 min read
Carbon allocation by individual plants gives us information on carbon cycling and sequestration by forests. How a plant invests its resources in different functions depends on the species and also the stress it might face during its life cycle. Plant response to stress and tasks they prioritize is an exciting field of study that looks deeper into the interaction between plants and their environment. Scientists use a simple metric—the leaf area—to tell them more about the inner physiology of the plant. The portable leaf area meter that is carried to fields comes in handy for measurements.
Resource allocation
Plants have a limited amount of resources that they must allocate to grow, reproduce, store, or develop defenses against herbivores and diseases.
Hence, it is crucial to understand how different species respond to various anthropogenic influences, such as rising temperatures and atmospheric carbon dioxide, or to natural stress like water, competition, herbivore, and pest attacks.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Carbon allocation has received the most attention due to its increase in the atmosphere in the last 100 years.
Carbon movement in plants is understood as a source-sink relationship. Plants source carbon from the environment for internal use. The leaves that conduct photosynthesis is the source of carbon in plants.
Plants use carbon for growth, reproduction, or defense. Some amount of carbon is also lost during respiration and root exudation.
A part of the carbon is used to produce non-structural carbohydrates,such as sugars or starches, and lipids. These are stored in sinks such as fruits, seeds, tubers, roots, or thicken the phloem/stems. In times of need, these sinks can be used by trees as a source of carbon; for example, deciduous trees use sinks in winter when they have no leaves to produce new carbon.
The allocation of resources to source and sink is a principle that is well known and exploited by agricultural scientists. They choose varieties that allocate less for vegetative growth (sources) and more to producing grains or fruits (sinks).
Stress Affects Resource Allocation
In favorable conditions, plants use carbon to grow and simultaneously form sinks. Stress, however, can change plants’ priorities.
Defoliation of trees can occur due to pests or the browsing of large mammals. Storms and frost are also some other factors that can defoliate trees.
As a result of defoliation, plants have to choose between allocating internal carbon to continue growing or for storage. The effects of defoliation on plants can also change with the intensity of defoliation. The following scenarios are possible:
1.) Source or carbon limitation occurs due to less availability of carbon; it can lead to
- reduction in both growth and storage.
- less growth, but carbon stores remain the same or decline.
2.) Sink limitation occurs due to the inability of the plant to grow because it cannot access carbon in its tissue. This limitation can occur because the sinks or reserves of non-structural carbon are less or lacking. In this case,
- plants opt to keep or increase carbon reserves to help them overcome more stress. So, the carbon stores increase, and growth decreases.
- if the sinks are adequate, plants can immediately prioritize growth.
There have been several studies on resource allocation in trees to understand how stress influences them. One of the most common metrics of growth that is measured by scientists in source-sink research is the leaf area.
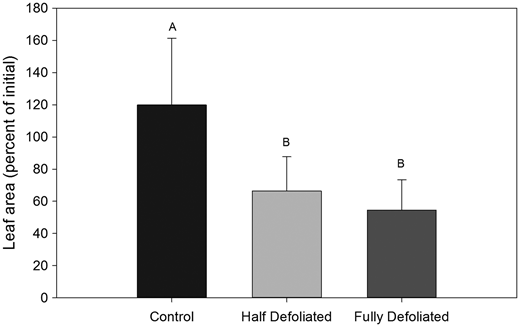
Figure 1: “Leaf area at harvest as a percent of initial leaf area.” (Image credits: https://academic.oup.com/treephys/article/33/11/1216/1708659)
Growth vs. Storage
How much of the plants’ resources are stored is an interesting question in itself. There is not much information on carbon pools in trees and plants, especially during or after recovery from stress. These traits in plants are likely to be genetically controlled, so it is essential to understand the plants’ response.
One study postulated that when the leaf loss is moderate, the trees may favor storage over growth, but when defoliation is severe, then both growth and storage will be reduced.
Scientists at the University of Pennsylvania carried out an on-campus experiment with two-year-old oaks, Quercus velutina saplings, to study short and long term effects of defoliation. Some oaks were entirely defoliated, while a second batch underwent half or no defoliation. Half defoliated trees had half their leaves removed.
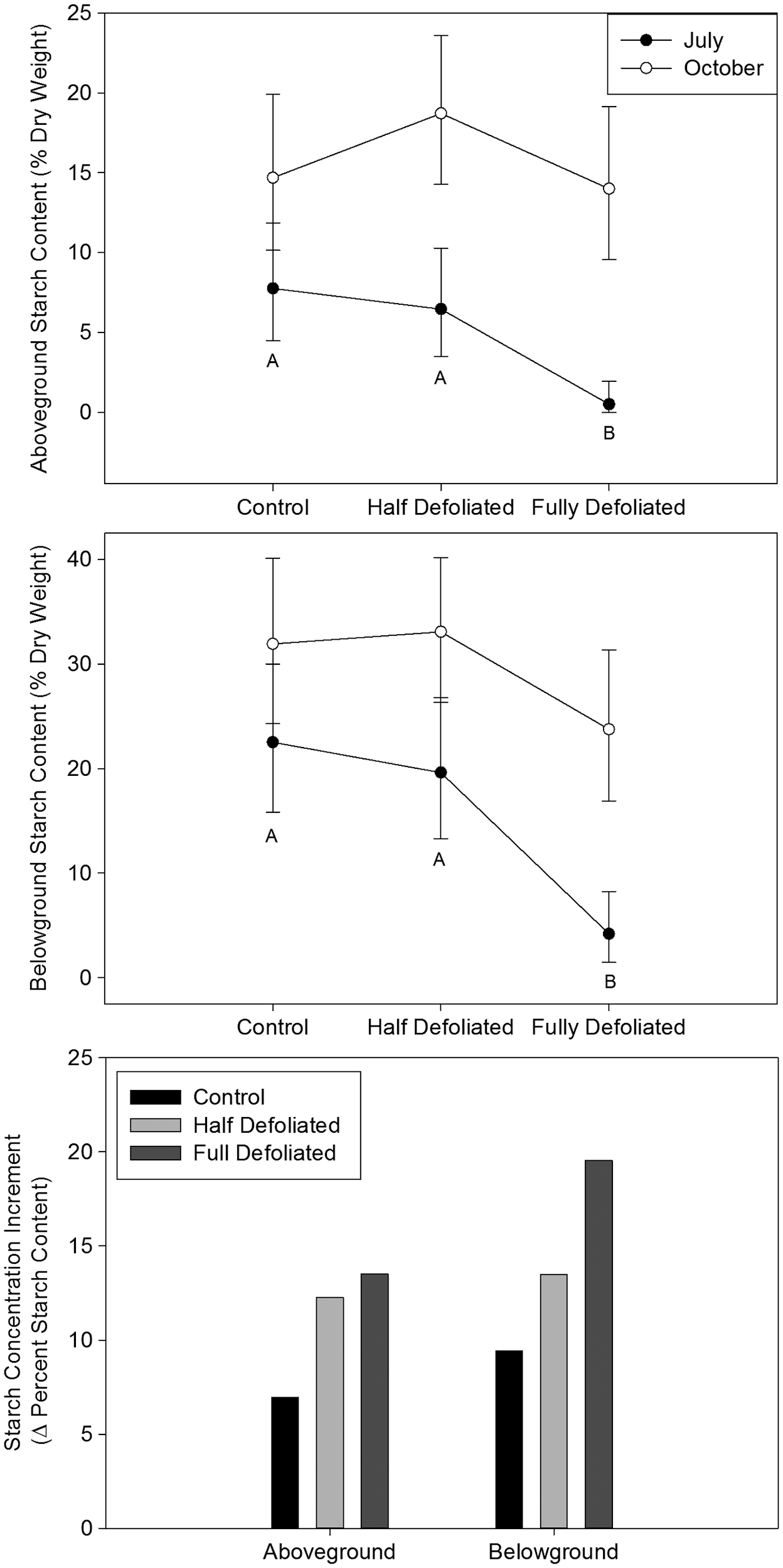
Figure 2: “Starch content of above- and below-ground woody tissue at July and October harvests. Starch concentration increment, a measure of allocation to storage, is the difference between back-transformed October and July average starch levels.” (Image credits: https://academic.oup.com/view-large/figure/25073241/tpt09304)
Total biomass of aerial and roots, along wuth leaf area changes, were used as metrics for growth and they were measured after three weeks and four months. Total biomass involved destructive sampling with the micro-oven method. Leaf area was measured non-destructively by the CI-203 Handheld Laser Leaf Area Meter, produced by the CID Bio-Science, Inc.
Roots and stems were weighed and analyzed for starch as the stored carbon metric.
Leaf level photosynthesis and nutrients like nitrogen content were also measured to study sink limitation.
The scientists found that growth was reduced in both half and entirely defoliated trees in the short and long-term. This is also reflected in the recovery of the leaf area, as shown in Figure 1. Both half and entirely defoliated trees had less leaf area than control plants. Defoliated trees also allocated more carbon for storage than control trees with no defoliation.
Half defoliated trees maintained their carbon stores in the short and long term. These results support the theory that trees are adapted to store carbon in times of stress, even at the expense of growth.
Entirely defoliated or severely stressed trees also had substantially reduced growth, and their carbon stores recovered only towards the end of the experiment. Initially, both leaf starch levels and root development were reduced in the first two weeks when there were no leaves to absorb carbon.
So, the scientists concluded that the oak saplings were suffering due to their inability to source carbon from the atmosphere and not due to sink limitation.
Storage vs. Reproduction
The same research group delved deeper into carbon allocation to see how the resource was split when reproduction was added to the options besides growth and storage.
This time, the experiment was conducted in the Rutgers University Pinelands Research Station in New Jersey. Six mature oaks (Quercus velutina Lam.) were entirely defoliated and compared to seven control oaks. The growth and carbon dynamics of both groups were followed for three years.
Radial growth of stems were used as growth parameters to calculate basal area. Leaves from the upper canopy were taken and measured using a CI-203 Handheld Laser Leaf Area Meter.
To measure carbon storage, the starch in leaves and wood cores from both stems and roots were used. Samples were then oven-dried after preparation.
To monitor reproduction, flowers and acorns were counted in two years.
Photosynthesis and nitrogen content at leaf level were measured after leaf regrowth to quantify sink limitation.
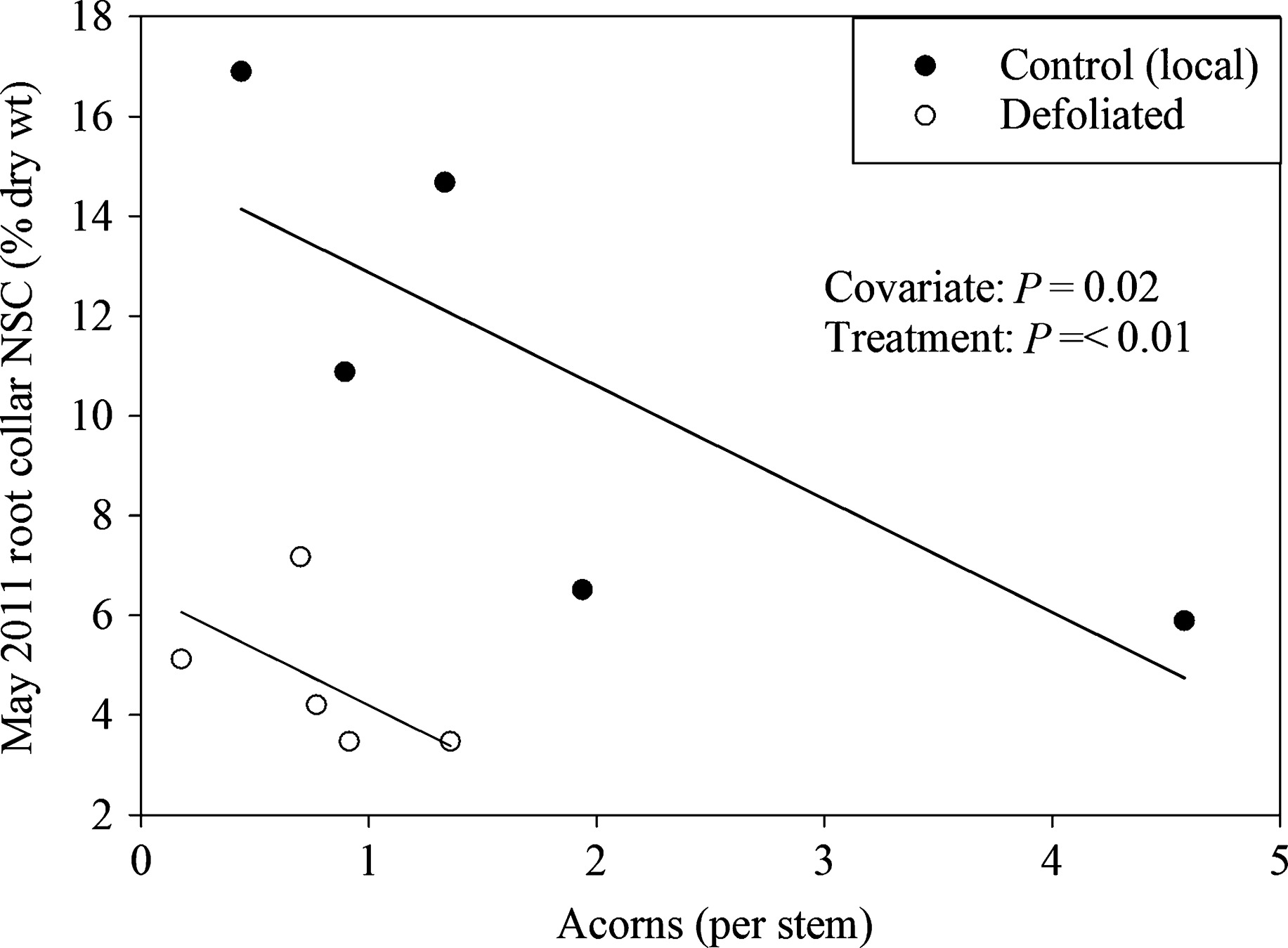
Figure 3: When more acorns are produced, there is less carbon stored. (Image credits: https://besjournals.onlinelibrary.wiley.com/doi/full/10.1111/1365-2745.12672)
Once again, after defoliation, the trees prioritized carbon storage above both growth and reproduction.
Growth was slower in all the years of observation, indicating that the tree was getting less carbon due to defoliation. The leaf area of the new flush was nearly half as big as the leaves on the control trees.
The trees continued to invest in producing acorns that were already formed, so ongoing reproductive cycles were also given priority over investing in new growth. However, the trees did not create fresh flowers or acorns but shifted the resources for storage; see Figure 3. Hence, new reproductive cycles suffered.
In the first year after defoliation, starch levels in trees were lower, as stored carbon was used to grow new leaves (see Figure 4 and Figure 5). Hence, the trees diverted the new carbon that they fixed to replenish their depleted stores so that, by the end of the second year, they were back to normal levels. Root carbon levels recovered faster than stem carbon.
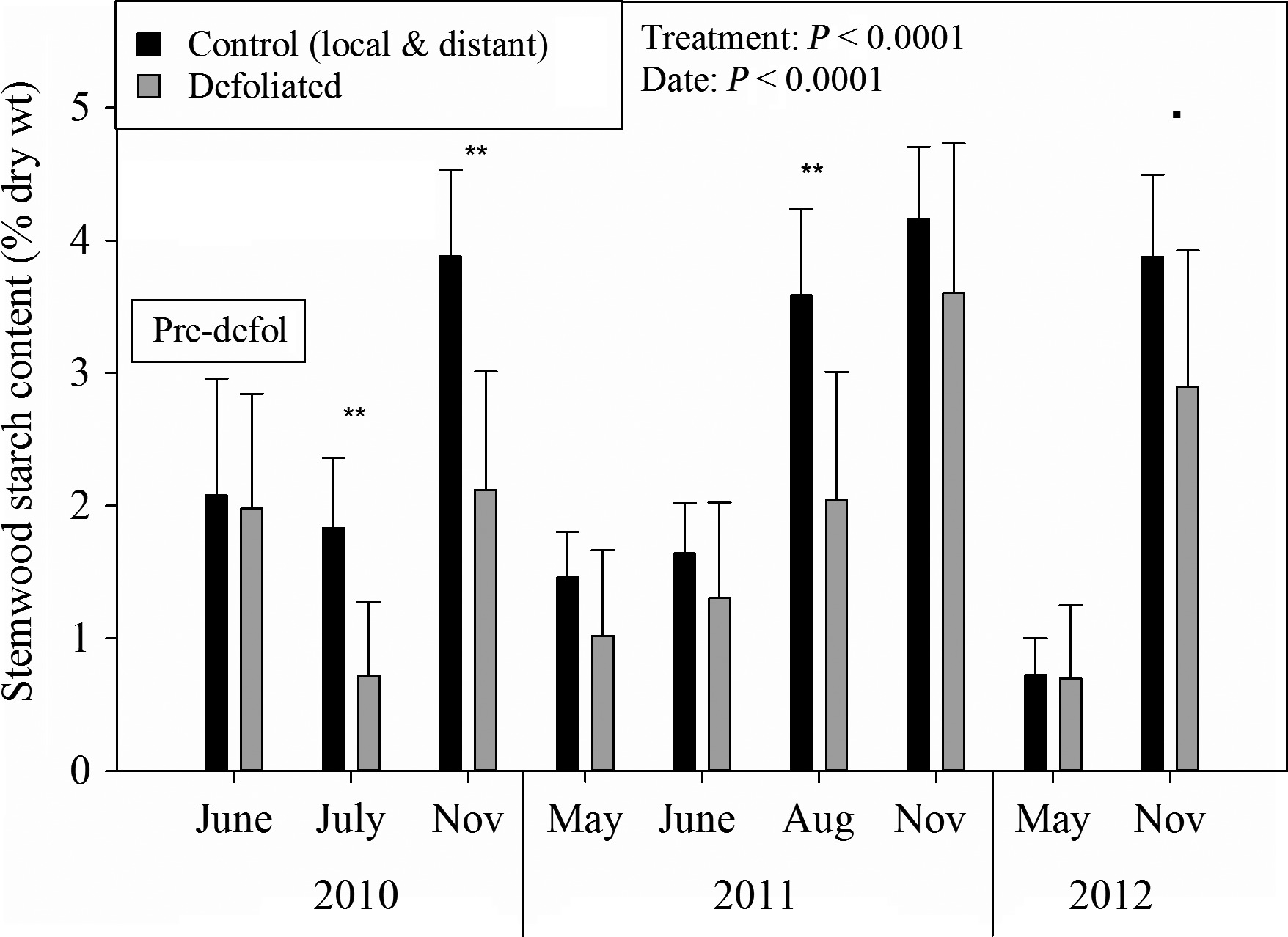
Figure 4: “Stemwood starch levels for defoliated and control trees.” (Image credits: https://besjournals.onlinelibrary.wiley.com/doi/full/10.1111/1365-2745.12672)
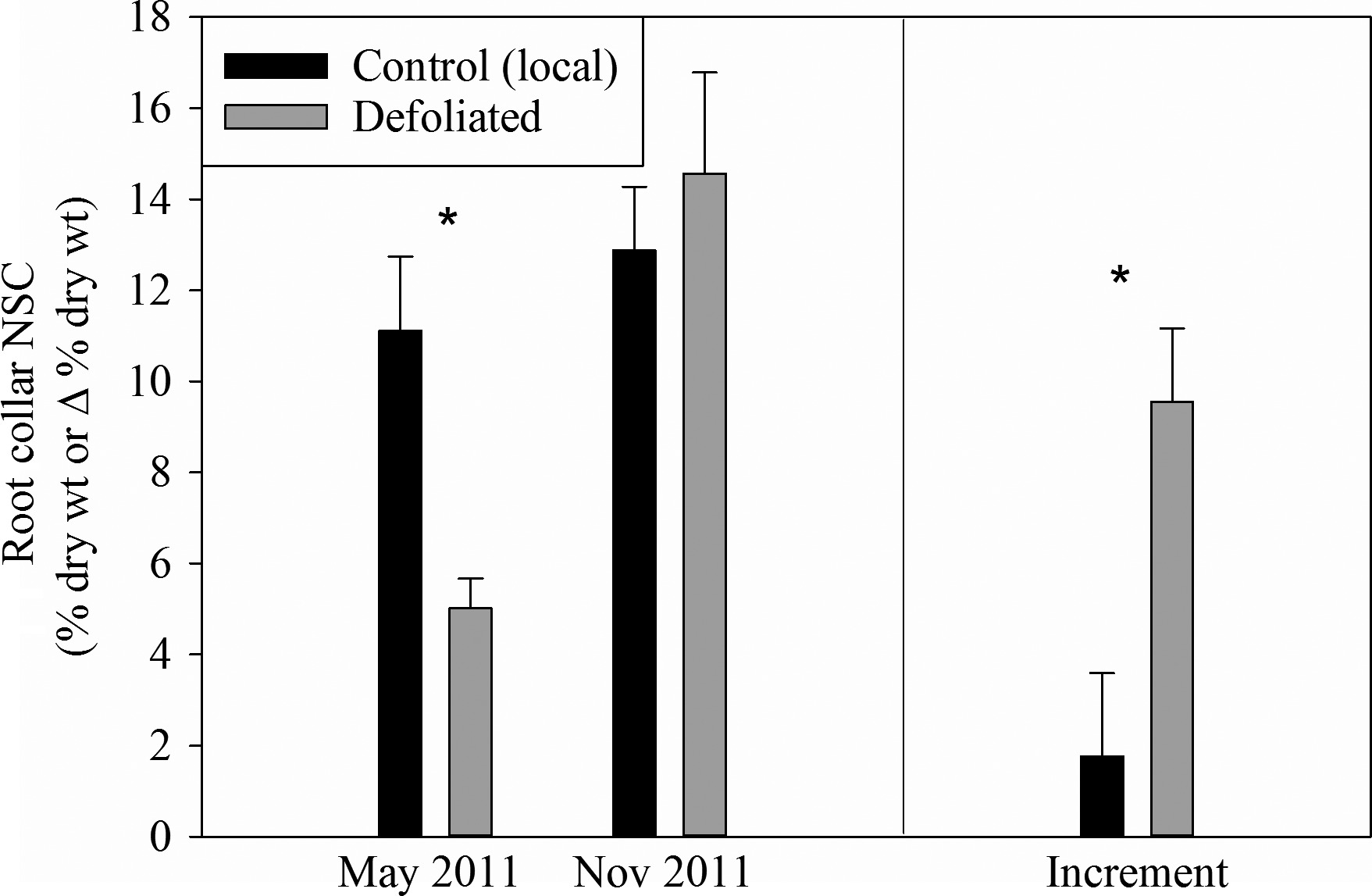
Figure 5: “Root carbon levels and increment the year following defoliation.” (Image credits: https://besjournals.onlinelibrary.wiley.com/doi/full/10.1111/1365-2745.12672)
In this case, the scientists concluded that after stress-induced defoliation, the trees do not start to grow again. The oaks prioritize storage so that their carbon reserves are adequate to maintain their fitness for future stress periods. This can be compared to people setting aside funds for future emergencies after having experienced one. It should be remembered that, in the case of trees, this is not a conscious choice. Rather, trees with this strategy have been the ones that have managed to survive and increase their number over centuries at the expense of trees that didn’t store enough carbon to withstand stress.
Demystifying Trees
The inner world of trees is fascinating. A simple instrument like the Handheld Laser Leaf Area Meter CI-203 can be used in the field to investigate a myriad of questions in forest sciences. This instrument gives results rapidly, within seconds, and can measure a range of leaf size and thickness to provide leaf width, length, perimeter, area, shape factor, aspect ratio, and void count. In this case, it has helped scientists understand how forests will respond to stress.
—
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature image courtesy of Rody09
Sources
Campany, C. E., Medlyn, B. E., & Duursma, R. A. (2017). Reduced growth due to belowground sink limitation is not fully explained by reduced photosynthesis. Tree Physiology, 37(8), 1042–1054. doi: 10.1093/treephys/tpx038
Hoch, G., Richter, A., & Korner, C. (2003). Non-structural carbon compounds in temperate forest trees. Plant, Cell and Environment, 26(7), 1067–1081. doi: 10.1046/j.0016-8025.2003.01032.x
Vanderklein, D., Cullen, A., & Belcourt, J.-E. (2015). Response of Japanese Barberry to Varying Degrees of Defoliation. Northeastern Naturalist, 22(2), 248–261. doi: 10.1656/045.022.0202
Wiley, E., Casper, B. B., & Helliker, B. R. (2016). Recovery following defoliation involves shifts in allocation that favour storage and reproduction over radial growth in black oak. Journal of Ecology, 105(2), 412–424. doi: 10.1111/1365-2745.12672
Wiley, E., Huepenbecker, S., Casper, B. B., & Helliker, B. R. (2013). The effects of defoliation on carbon allocation: can carbon limitation reduce growth in favour of storage? Tree Physiology, 33(11), 1216–1228. doi: 10.1093/treephys/tpt093
White, A. C., Rogers, A., Rees, M., & Osborne, C. P. (2015). How can we make plants grow faster? A source–sink perspective on growth rate. Journal of Experimental Botany, 67(1), 31–45. doi: 10.1093/jxb/erv447
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- Plant Respiration: Its Importance and Applications
- The Forest Canopy: Structure, Roles & Measurement
- Stomatal Conductance: Functions, Measurement, and…
- Forest & Plant Canopy Analysis – Tools…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- 50 Best Universities for Plant Science






