October 21, 2020 at 9:35 pm | Updated March 16, 2022 at 11:37 am | 8 min read
Drought is the most common stress and has the largest effect on the growth and production of crops. Nearly a fourth of agricultural areas worldwide are affected by water shortage. Plants adapt to drought by altering their genetics, physiology, and biochemistry. Hence, these traits are being used to screen new cultivars, which can sustain growth and yield during water stress. The relevance of these traits can vary between different species and have to be tested individually for crops.
Effects of Drought on Plants
Drought affects both aboveground and underground parts of a plant. It affects growth, functioning, flowering, and seed set.
Biochemical responses are the first to occur when a plant experiences drought. They secrete hormones to signal water stress to their organs. These, in turn, adjust physiology and produce chemicals to respond better to drought.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Physiologically, drought decreases photosynthesis, transpiration, and stomatal conductance. It can also increase the generation of reactive oxygen species (ROS) or alter resource allocation to different organs resulting in morphological or phenological changes.
Morphological changes in size and growth of leaves, stems, and roots are common during drought.
The responses and underlying causes are hard to predict. Consequently, scientists are including a growing number of traits to find drought-resistant or tolerant varieties.
Morphological and Physiological Drought Response in Lentils
Lentils (Lens culinaris) form an important source of proteins in India for people and cattle alike, but farmers were not sure which stage of the crop was most sensitive to drought.
Hence, scientists decided to answer this question for two lentil varieties: small-seeded HUL-57 and large-seeded IPL-406. They checked the morphological and physiological drought response in each life stage of the lentils. They also tested the validity of drought indices, drought tolerance efficiency (DTE), and geometric mean productivity (GMP) to measure the drought response of the crop.
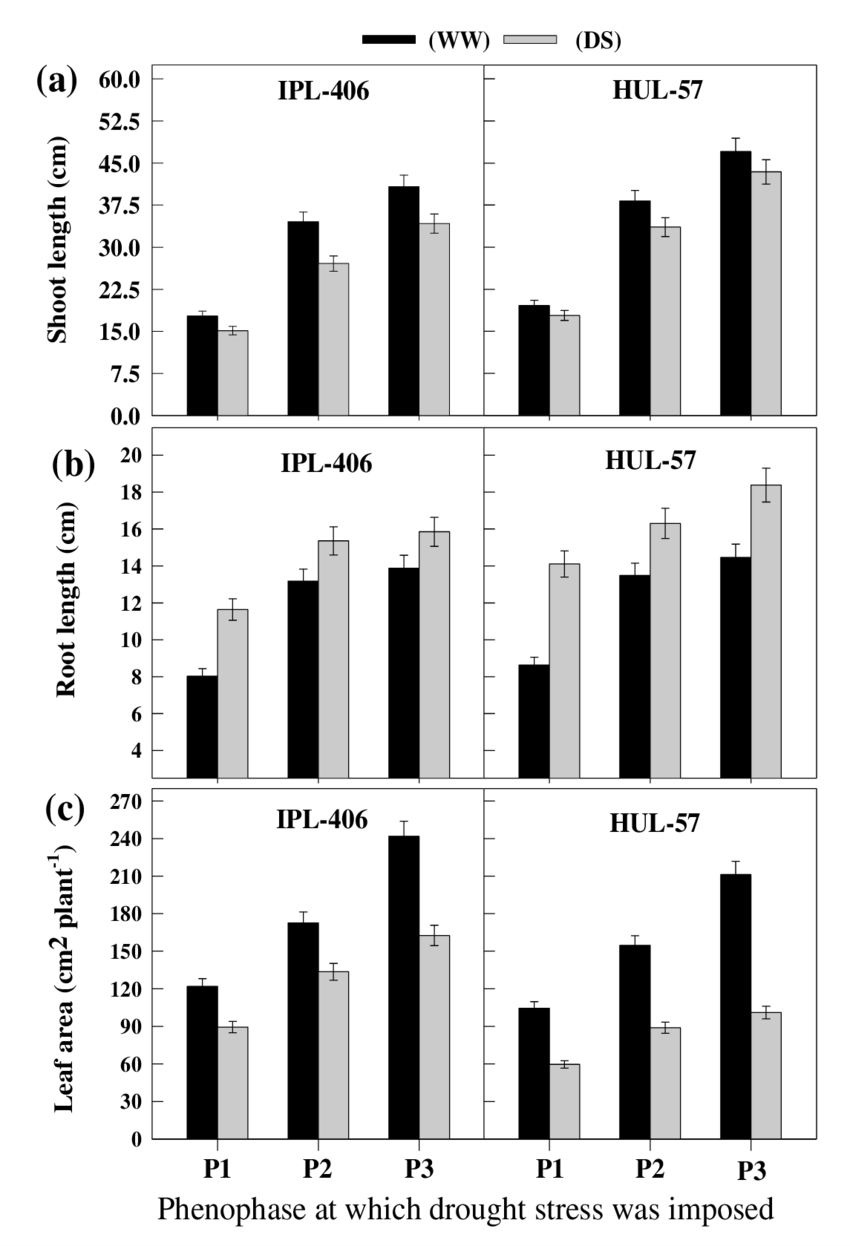
Figure 1. “(a) Shoot length (cm), (b) Root length (cm), (c) Leaf area (cm2 plant-1) in two genotypes of lentil under well-watered (WW) and drought stress (DS) subjected at specific phenophase of growth,” Mishra et al., 2018. (Image credits: Int.J.Curr.Microbiol.App.Sci (2018) 7(1): 2288-2304).
Each cultivar was grown in a hundred and fifty pots. Twenty-five of each variety were given 75% of available moisture and were used as control. Twenty-five pots each were subject to drought or moisture at 30% during the three phenological stages: mid-vegetative, flower initiation, and pod-formation. Observations were made at permanent wilting point. Subsequently, plants were given normal levels of water until harvest.
At all three stages during drought and after recovery, the morphological parameters observed were shoot and root length, as well as leaf area.
Root length was measured by de-potting the plants and carefully separating and cleaning roots.
To measure leaf area, the scientists used the CI-202 Portable Laser Area Meter, produced by CID BioScience Inc. It is a handheld device that measures the area of leaves non-destructively and rapidly, with a resolution of 0.01 cm2. The CI-202 can save 8000 measurements that can be transferred to a computer later for easy analysis.
Physiological parameters measured at the end of the drought episode were the relative water content, cell membrane injury due to temperature and osmotic stress, and dry matter production and nitrogen content of various plant parts.
Shoot length was decreased at all the three phenological stages for both cultivars. HUL-57, which manages to maintain turgor even during drought, suffers less.
The scientists attribute the decrease to an impact on cell division and expansion due to water deficiency.
These physiological processes were also responsible for the reduction in leaf area in both cultivars during all life stages. The total leaf area of the plant is also affected by the senescence of leaves due to drought. However, the leaves in HUL-57 were smaller than IPL-406.
Root length was more developed in plants that were stressed by drought. HUL-57 roots always had deeper roots at all stages than IPL-406. Plants that have longer roots have a better ability to reach and absorb more water, so they are less affected by drought.
The increase in root length was due to more allocation of dry matter during the flowering stages. During vegetative stages, leaves received more dry matter in both cultivars. Biomass and nitrogen accumulated before flowering was sent to developing pods.
Cell membrane was affected by both heat and osmotic stress, in all phenological stages, but to a lesser extent in HUL-57. So, this cultivar suffered less leaching of cell solutes.
During stress, plants that had less leaf area had better relative water content and cell membrane stability in leaves. Plants with better water content also showed more cell membrane stability.
The effect of drought was less during the vegetative stage, followed by the flowering stage. The pod-filling stage in both cultivars was most affected by drought. HUL-57 suffered less due to drought than IPL-406, as it was better adapted for dry conditions morphologically and physiologically.
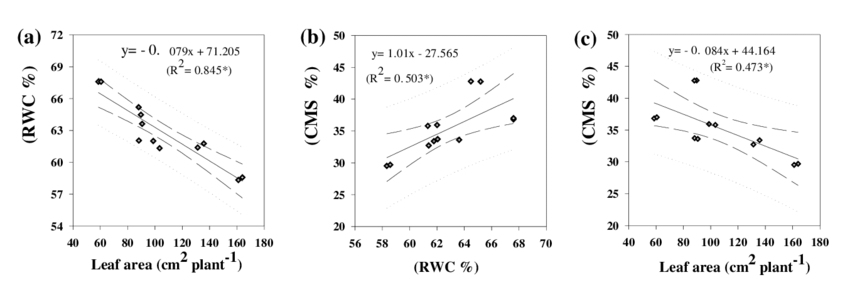
Figure 2: “The relationship between (a) leaf area and relative water content of leaves; (b) relative water content of leaves and cell membrane stability; (c) leaf area and cell membrane stability,” Mishra et al., 2018. (Image credits: Int.J.Curr.Microbiol.App.Sci (2018) 7(1): 2288-2304).
Both the drought indices decreased in drought-stressed plants and are recommended for use to screen new cultivars in lentils; however, GMP was more sensitive than DTE.
Changing Genetic Makeup for Drought Tolerance
Changes in all plant parameters—physiological, biochemical, or morphological—are controlled by genes. So, some scientists have decided to use genetic engineering to produce drought-tolerant crops.
In one such experiment in China, genes from a common weed were introduced into Danshen or Salvia miltiorrhiza (Lamiaceae), a traditional Chinese medicinal herb, to drought-proof the latter.
Danshen, which is used to treat cardiovascular diseases, blood circulation disorders, and inflammation, is extensively cultivated. However, intensive agricultural methods and drought have been lowering their yields.
Arabidopsis thaliana (Brassicaceae), a small flowering weed, is used as a model plant for genetic and molecular biology experiments. The whole genome of the plant was published twenty years ago and the transcription factor AtDREB1A from A. thaliana has been successfully used in other gene manipulations.
Due to drought, plants produce dehydration response elements (DREs), which interact with proteins that bind them (DREB). These DREB proteins control the expression of many genes involved in stress response; hence, many genetic experiments target these transcription factors.

Figure 3: “Agrobacterium-mediated transformation and transgenic plant regeneration. (A) Induction of bar-resistant calli. (B) Differentiation of bar-resistant calli. (C) Formation of bar-resistant shoots. (D) Rooting of bar-resistant plantlets. (E, F) Transplanting of positive transformants,” Wei et al., 2016. (Image credits: https://academic.oup.com/pcp/article/57/8/1593/2755829).
AtDREB1A was introduced into two gene promoters, RD29A and CaMV 35S, involved in drought response in Danshen. Agrobacteruim was used as the medium to transfer the new engineered genes into the Danshen leaf bits or calli. The new transgenic lines were replicated in a medium, as shown in Figure 3.
From the two genes, transgenic lines of pRD29A::AtDREB1A line 17 (A-17), pRD29A::AtDREB1A line 31 (A-31), and one line of p35S::AtDREB1A-18 (A-18) were produced.
The effect of AtDREB1A on the metabolism of the three new Danshen lines was assessed by studying the transcriptomic changes, photosynthesis, and antioxidative mechanisms after they were subject to eleven days of drought along with wild-type plants for comparison.
Shoot growth was monitored throughout the stress. For three days, all plants grew well. By nine days, wild plants and A-18 had withered. The A-17 and A-31 lines were unaffected at this stage, but wilted after eleven days of drought. After eleven days, all wilting plants were watered. The transgenic lines recovered, and lines A-17 and A-31 recovered faster.
After nine days of drought, the relative water content, chlorophyll content, and gas exchange of plants were tested.
The scientists used the CI-340 Handheld Photosynthesis System, manufactured by CID Biosciences Inc., to non-destructively record rates of photosynthesis, stomatal conductance, and transpiration. The tool measured gas exchange by an Infrared gas analyzer. Researchers used additional modules to control and maintain light intensity at more than 1,200 μmol m−2 s−1 and CO2 at 400 ppm during all the tests.
The scientists also checked the antioxidative metabolism by measuring malondialdehyde (MDA) concentrations to determine lipid oxidation and the amount of antioxidant enzymes, superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT).
All transgenic plants showed better relative water content than wild plants under drought. A-17 and A-31 had higher values than A-18.
Under well-watered conditions, gas exchange processes were similar. However, under drought stress, the rate of photosynthesis, stomatal conductance, and transpiration were severely reduced in wild plants, while transgenic lines were less affected. Once again, A-17 and A-31 performed better than A-18. See Figure 4.
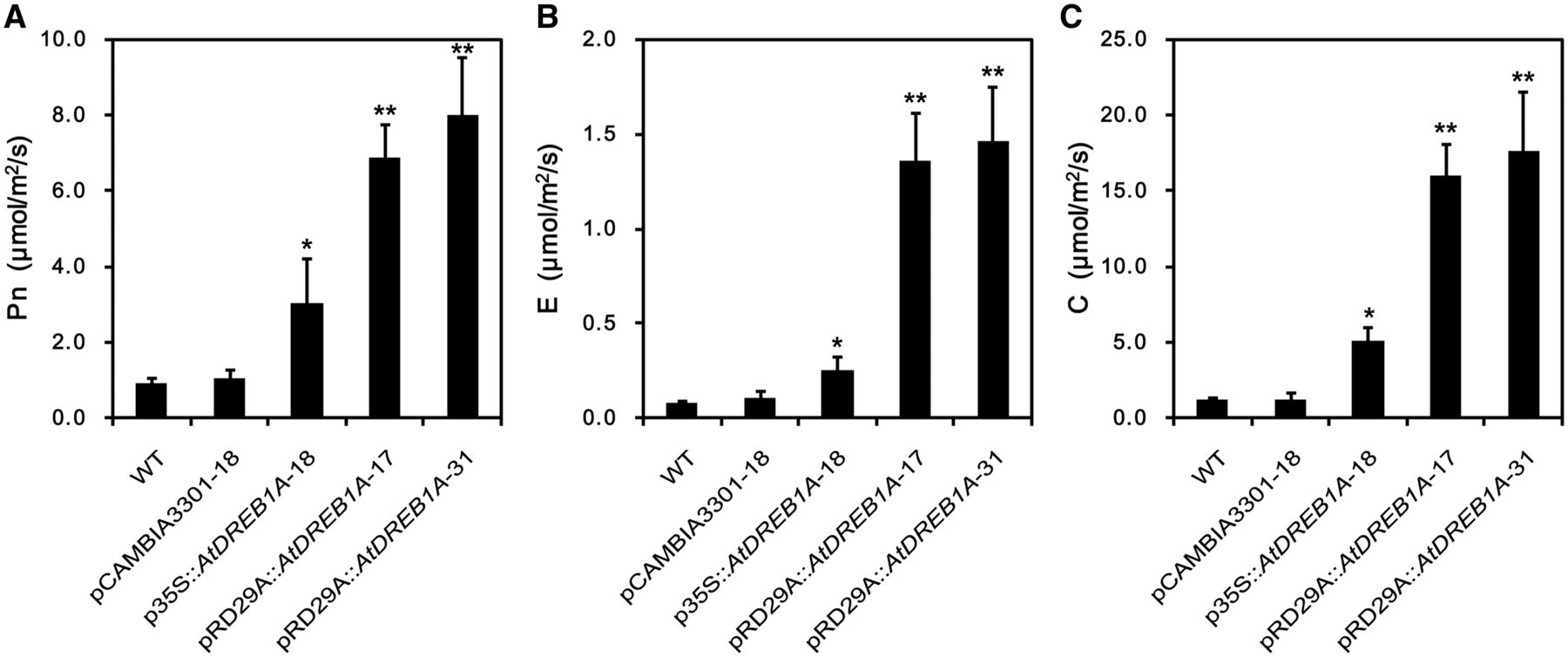
Figure 4: “Effects of drought stress on leaf gas exchange parameters in WT and transgenic Salvia miltiorrhiza plants following dehydration for 9 d. (A) Net photosynthesis rate (Pn); (B) transpiration rate (E); (C) stomatal conductance rate (C). WT, wild type,” Wei et al. 2016. (Image credits: https://academic.oup.com/pcp/article/57/8/1593/2755829)
The biochemistry of the plants was improved in transgenic lines as they produced more antioxidants SOD, POD, and CAT necessary to fight ROS produced by stress, with slightly higher levels in A-17 and A-31 followed by A-18.
MDA content decreased in transgenic lines as a response to drought, with the lowest rates found in A-17 and A-31, then A-18 in comparison to wild plants.
The pharmaceuticals of interest in Salvia, salvianolic acids, and tanshinones, which were estimated five months after plant maturation, were higher in the transgenic lines. A-18 produced more salvianolic acids and A-31 more total tanshinones; A-17 was not tested.
Hence, the Chinese scientists concluded that introducing the transcription factor DREB1A/CBF3 increased the drought tolerance of S. miltiorrhiza by regulating genes necessary to fight stress and improve secondary metabolites production.
Multiple Strategies
There are various methods by which drought stress can be alleviated in new crop varieties. For this, the scientists have to first find out the relevant parameter to alter, find ways to do it, and to evaluate the performance of cultivars. Sophisticated experiment designing that is required in all these steps has to go hand-in-hand with relevant technology that can conduct analysis in the field. Thus, the scientific tools manufactured by CID BioScience make an important contribution to research to ensure food security.
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature image courtesy of NASA Goddard Space Flight Center
Sources
Fathi, A., & Tari, D. B. (2016). Effect of Drought Stress and its Mechanism in Plants. International Journal of Life Sciences, 10(1), 1-6. doi:10.3126/ijls.v10i1.14509
Koornneef, M., & Meinke, D. (2010). The development of Arabidopsis as a model plant. The Plant Journal, 61(6), 909-921. doi:10.1111/j.1365-313x.2009.04086.x
Mishra, B., Srivastava, J., & Lal, J. (2018). Drought Resistance in Lentil (Lens culinaris Medik.) in Relation to Morphological, Physiological Parameters and Phenological Developments. International Journal of Current Microbiology and Applied Sciences, 7(1), 2288-2304. doi:10.20546/ijcmas.2018.701.277
(n.d.). About Arabidopsis. Retrieved June 16, 2020, from https://www.arabidopsis.org/portals/education/aboutarabidopsis.jsp
Plant Transformation Using Agrobacterium tumefaciens – ABNE: African Biosafety Network of Expertise. (n.d.). Retrieved June 16, 2020, from http://nepad-abne.net/biotechnology/process-of-developing-genetically-modified-gm-crops/plant-transformation-using-agrobacterium-tumefaciens/
Wang, L., Ma, R., Liu, C., Liu, H., Zhu, R., Guo, S., Tang, M., Li, Y., Niu, J., Fu, M., Gao, S., & Zhang, D. (2017). Salvia miltiorrhiza: A Potential Red Light to the Development of Cardiovascular Diseases. Current pharmaceutical design, 23(7), 1077–1097. https://doi.org/10.2174/1381612822666161010105242
Wei, T., Deng, K., Liu, D., Gao, Y., Liu, Y., Yang, M., . . . Zhang, Y. (2016). Ectopic Expression of DREB Transcription Factor, AtDREB1A, Confers Tolerance to Drought in Transgenic Salvia miltiorrhiza. Plant and Cell Physiology, 57(8), 1593-1609. doi:10.1093/pcp/pcw084
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- The Forest Canopy: Structure, Roles & Measurement
- Plant Respiration: Its Importance and Applications
- Forest & Plant Canopy Analysis – Tools…
- Stomatal Conductance: Functions, Measurement, and…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- Irrigating with Saline or Seawater






