December 18, 2020 at 5:07 am | Updated December 18, 2020 at 5:07 am | 6 min read
Maximizing photosynthesis is key to increasing food yield and remains one of the top priorities in both the breeding and plant science world. Measuring photosynthesis in crops in field conditions is necessary, and it is, indeed, the ideal way to test new varieties and agricultural practices. However, until recently, difficulty in performing field measurements has been a major hurdle in improving photosynthetic efficiency.
Can Manipulating Agronomic Practices Improve Fruit Yields?
A group of scientists from Chile, Andrade et al., wanted to test the effect of thinning fruits on photosynthesis in early and late-harvested nectarines and peaches during fruit development.
Thinning is common in commercial orchards. It involves removing some fruits to reduce the number of sinks for sugars produced by photosynthesis so that the remaining fewer fruits can become bigger and sweeter. However, if fruits thinning is too great, it leads to an accumulation of sugars in leaves, which can reduce the demand for photosynthesis.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
The researchers experimented on the mid-season variety “Magique” and late-season “Red Pearl.” They needed to test photosynthesis, stomatal conductance, transpiration, chlorophyll fluorescence, and sugar content in leaves.
There were three experiments: total fruit removal, light/commercial fruit removal, and no thinning. Leaves on four branches of each treatment were tested, so at each reading, the scientists had to make 12 sets of tests. These tests were conducted throughout the fruiting season.
They needed instruments that were accurate, easy to use, and portable.
Why is Measuring Photosynthesis Challenging?
There are many methods and protocols to measure photosynthesis, as it has always been an interesting and crucial topic of research, given how central photosynthesis is to life on earth.
Photosynthetic rate is measured by the following gas exchanges happening through stomata:
- Absorption and use of carbon dioxide (CO2) from the air by leaves
- Production of oxygen (O2), one of the end products of the process
Photosynthesis can also be measured by estimating dry matter accumulation and the production of carbohydrates. While these two methods involve in vitro laboratory tests, gas exchanges are also performed in situ in the field.
Measuring photosynthesis in the field is not easy for many reasons.
During gas exchange measurements, there are a great number of external environmental factors that can influence the process. This abundance of potentially interfering factors is part of what makes estimating photosynthetic rate so difficult.
For example, stomatal conductance and the operation of Photosystem II (PSII) can be influenced by variations in light and temperature. The heterogenous nature of light in the field means this is an ever-present hurdle in in situ measurement.
These factors can be maintained and controlled in a laboratory or greenhouse; therefore, several photosynthetic experiments are conducted indoors. However, environmental conditions in greenhouses are not the same as those in the field, so field replication is necessary.
It is well known that measuring photosynthesis in vitro, with a detached leaf, gives estimates that are very different from those made in situ on the field, with the leaf still attached to the plant or tree. In vitro measurements of photosynthesis can be up to 40% lower than in situ measurements.
The disruption of the leaves’ water supply from the roots is one of the main reasons for the lower estimated values, as water is a crucial ingredient in photosynthesis. Additionally, a water deficit in leaves could partially or fully close the stomata, cutting the intake of carbon dioxide. PSII’s sensitivity to water deficiency further complicates this process.
Measurements of photosynthesis in trees are also complicated by height. So, scientists have tried using leaves attached to branches, but even here water supply is often still limited. Cracking the broken stem can increase water supply and improve photosynthesis, but this is no replacement for in situ studies.
In experiments and trials, it can be necessary to perform large-scale measurements repeatedly over the crop cycle, and neither in vitro, nor greenhouse measurements serve the purpose of accurately estimating photosynthetic efficiency.
Most conventional equipment used for photosynthetic measurement is large and involves several disparate components. This makes their use in the field often impractical or impossible given the scale of most thorough studies.
Moreover, photosynthetic measurements must be made within a certain time window to ensure uniform light conditions. So, the scientists, Andrade et al., needed a method of estimation that was also rapid otherwise the tests would have to be spread over many days, which, again, introduces heterogeneity.
Scientific Solution: The CI-340 Handheld Photosynthesis System
The scientists found a solution to deal with the problems of accuracy, speed, and equipment size in measuring photosynthesis. They chose to use the CI-340 Handheld Photosynthesis System, produced by CID Bio-Science Inc. It is an Infra-Red Gas Analyzer (IRGA) that is portable, light, and can be used single-handed. The CI-340, which can also measure stomatal conductance and transpiration, is designed for field use, so it circumvents the problems faced by in vitro estimations.
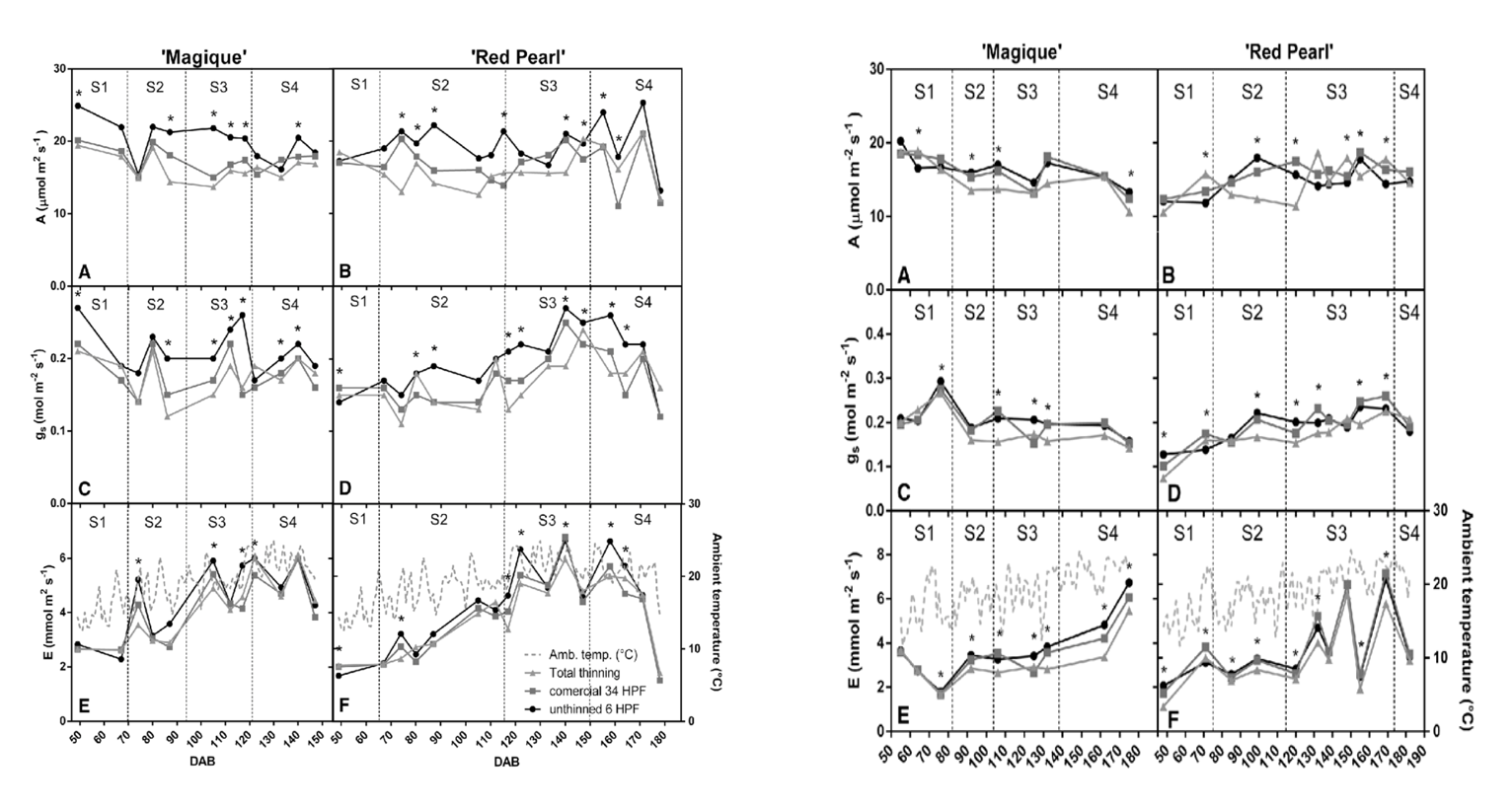
Figure 1 a. and 1 b.: “Changes in photosynthetic rate (a, b), stomatal conductance (c, d), transpiration in ‘Magique’ and ‘Red Pearl’ trees (e, f), and average ambient temperature (f) in the first season were evaluated in 2013-2014 (Figure 1 a) and in the second season in 2014-2015 (Figure 1 b). Measurements were performed in different thinned trees (• = unthinned, h = commercial thinning, and ∆= completely thinned),” Andrade et al. 2019. (Image credits: Theoretical and Experimental Plant Physiology, 31(2), 341-356. doi:10.1007/s40626-019-00150-0)
The CI-340 estimates photosynthetic rate as the rate of CO2 assimilation by measuring the CO2 levels in the air entering and leaving the leaf chamber.
The transpiration rate is estimated by measuring water vapor in the air entering and leaving the leaf chamber to calculate the rate of water vapor flux for a one-sided leaf area. The CI-340 records stomatal conductance by measuring the transpiration rate as a function of leaf temperature.
The tool consists of a leaf chamber that can be connected directly to the gas analyzer for rapid readings. Individual CO2 level readings take less than half a minute. There are ten leaf chambers to choose from to fit leaves and needles of various sizes.
The CI-340 is suitable for measuring photosynthesis, both in an open and closed system, giving scientists more control. In the open system, air entering the leaf chamber comes from the surrounding air or control modules and is allowed back into the atmosphere. In the closed system, the air is recirculated back into the leaf chamber.
Benefits During the Experiment
With the CI-340, the scientists were able to easily measure net photosynthetic rate, stomatal contuctance, and transpiration rate once a week during the entire fruiting season. Over the course of the experiment a total of 132 tests were taken—12 tests on each of 11 occasions.
The CI-340 is an asset in experiments where scientists are trying to control all factors and test only the effects of thinning, as it is able to reduce environmental heterogeneity while maintaining field conditions. This way, scientists can be more confident that the results they see are indeed the effects of the factors they are manipulating. The CI-340 has various accessories that can be used with the leaf chamber to control all the environmental factors that influence photosynthesis, such as light intensity, CO2, H2O, and temperature.
There is also a module that allows scientists to measure chlorophyll fluorescence and photosynthesis simultaneously to understand the changes that occur at the physiological level.
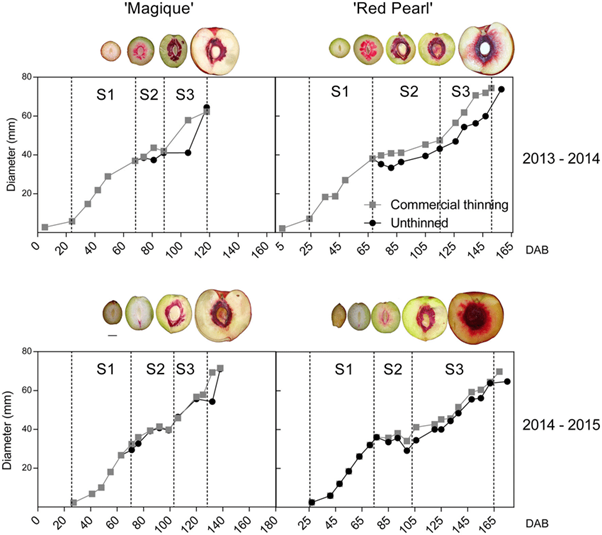
Figure 2: “Magique’ and ‘Red Pearl’ fruit development dynamics during the two seasons. Three different stages of development were identified: (1) S1: 1st stage of growth due to cellular proliferation; (2) S2: cessation of growth due to pit lignification; and (3) S3: 2nd phase of growth due to cell elongation,” Andrade et al. 2019. (Image credits: Theoretical and Experimental Plant Physiology, 31(2), 341-356. doi:10.1007/s40626-019-00150-0)
The scientists found that thinning decreased stomatal conductance, as expected, due to sugar accumulation in leaves, leading to a decrease in photosynthesis in both varieties. Increased photosynthesis was seen in trees that had more fruits and no thinning.
The reduction in photosynthetic rate due to commercial thinning did not affect the fruit size and quality compared to leaving all fruits on the trees in “Magique,” the early-season variety. However, thinning did improve the size of the late-harvest variety “Red Pearl” and also increased the development of root suckers in this variety.
Get Reliable Results
The processes and scientific principles behind fruit yield fluctuations can be complex. However, the availability of handheld portable tools, like the CI-340 Handheld Photosynthesis System, can make the study itself simple.
The scientists were able to monitor several intricate internal processes, including photosynthesis, at the physiological level through analysis in the field. These tests were expeditiously and accurately performed with the CI-340. In this case, the scientists could advise farmers to spare time and effort in thinning the early-variety and limit the practice only to late-harvest peaches to increase their return on investment.
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature image courtesy of Rosario Navarrete Rodriguez
Sources
Andrade, D., Covarrubias, M. P., Benedetto, G., Pereira, E. G., & Almeida, A. M. (2019). Differential source–sink manipulation affects leaf carbohydrate and photosynthesis of early- and late-harvest nectarine varieties.
Theoretical and Experimental Plant Physiology, 31(2), 341-356. doi:10.1007/s40626-019-00150-0
Measuring the rate of photosynthesis. (n.d.). Retrieved November 04, 2020, from https://www.saps.org.uk/secondary/teaching-resources/157-measuring-the-rate-of-photosynthesis
Meng, C., Liu, X., Chai, Y., Xu, J., & Yue, M. (2019). Another choice for measuring tree photosynthesis in vitro. PeerJ, 7, e5933. https://doi.org/10.7717/peerj.5933
Tian-Gen, C., Chang-Peng, X., Ming-Nan, Q., Hong-Long, Z., Qing-Feng, S., & Xin-Guang, Z. (2017). Evaluation of Protocols for Measuring Leaf Photosynthetic Properties of Field-Grown Rice. Rice Science, 24(1), 1-9. doi: 10.1016/j.rsci.2016.08.007
Walker, B. J., Busch, F. A., Driever, S. M., Kromdijk, J., & Lawson, T. (2018). Survey of Tools for Measuring In Vivo Photosynthesis. Methods in Molecular Biology Photosynthesis, 3-24. doi:10.1007/978-1-4939-7786-4_1
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- Plant Respiration: Its Importance and Applications
- The Forest Canopy: Structure, Roles & Measurement
- Stomatal Conductance: Functions, Measurement, and…
- Forest & Plant Canopy Analysis – Tools…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- 50 Best Universities for Plant Science






