October 14, 2020 at 3:26 am | Updated March 14, 2022 at 1:09 pm | 7 min read
Photochemical Reflectance Index (PRI) can be measured remotely to estimate several types of stress and nutrient deficiency in plants. It has been shown that PRI is an indicator of photosynthetic efficiency, which changes when plants face stress. Portable but accurate spectrometers that can make easy and rapid measurements of leaf spectral interactions help in establishing the science that is being applied in precision and smart farming.
PRI’s Association with Photosynthesis
When plants are exposed to stress—such as high solar or ultraviolet radiation, heat, drought, salt, chilling, heavy metals, air pollutants, mechanical stress, pathogen attack, and nutrient deficiency—there is an increase in accumulation of Reactive oxygen species (ROS). The level of ROS is an indicator of stress in plants and animals.
ROS are produced by electron transport chains. In plants, the most important are those that occur during photosynthesis, photorespiration, and NAD(P)H oxidases–peroxidases at cell walls.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
The excitation of chlorophyll in Photosystem II (PSII) during photosynthesis produces ROS that can photodegrade the photosynthetic apparatus.
Plants try to scavenge ROS to prevent the damage they cause. One such mechanism is the xanthophyll cycle, which is known to protect plants from stress caused by chilling, light intensity, drought, heat, senescence, and salinity.
Non-enzymatic antioxidants produced during the de-epoxidation processes of the xanthophyll cycle act against ROS. The conversion of epoxidized xanthophyll (violaxanthin) to de-epoxidated xanthophyll (antheraxanthin and zeaxanthin) is light dependant and happens during high light intensity. Zeaxanthin binds with proteins resulting in non-photochemical quenching (NPQ) that dissipates excitation energy.
By absorbing the excess light not being used by the photosynthetic apparatus, the xanthophyll cycle prevents damage to leaves.
The change in pigments in the xanthophyll cycle can be measured by changes in reflectance at red 531nm and red 570nm, and it is called Photochemical Reflectance Index (PRI).
PRI is closely related to the PSII efficiency. Hence, PRI can be used as a measure of carbon dioxide intake not only at the leaf level but also on the canopy scale. Therefore, satellite imagery uses PRI to collect information on the health and nutrition status of plants in precision and smart farming.
It is difficult to calculate chlorophyll fluorescence that is the direct measure of PSII efficiency, so foliar PRI measurement—which is rapid, non-destructive, and easy—is used as a remote method of estimating photosynthetic estimation.
Effects of Chilling on PRI
Let us consider chilling as an example to understand how the PRI monitors stress.
Due to chilling, the plant is stressed, and the rate of photosynthesis is reduced. Therefore, much of the light absorbed by the leaves is not used, and the efficiency of the PSII is lowered. This results in an increase in xanthophyll de-epoxidation. The duration of the effect of chilling stress is, however, not clear.
Scientists wanted to check how long the effects of chilling would last in tropical species like mango (Mangifera indica L), as they are sensitive to low temperatures that exist in temperate countries like Japan where the fruit cultivation is being introduced.
JH Weng and his associates from the National Chung-Hsing University, Taichung, Taiwan, tested mango in the winter (February) and spring (March) when temperatures are around 12-15 degrees Celsius. In sub-temperate regions, cold mornings are associated with bright sunshine, so the scientists tried to duplicate these conditions.
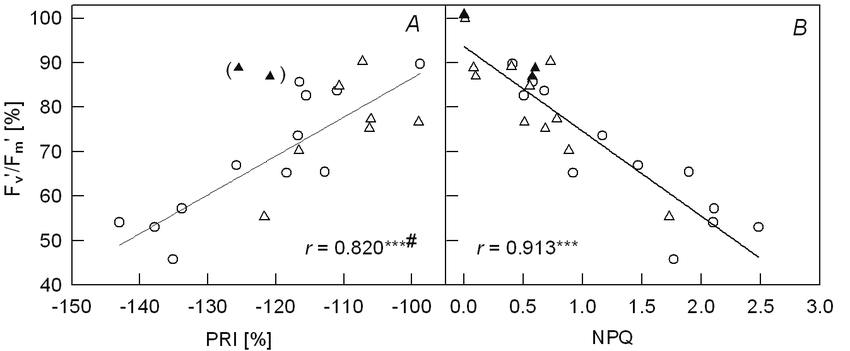
Figure 1. “Relationships between efficiency of energy conversion of PS2 (F v ‘/F m ‘, F v /F m before treatment as 100 %) and relative PRI [photochemical reflectance index, calculated from leaf spectral reflectance, = (R 531 – R 570 )/(R 531 + R 570 ), before treatment as –100 %], and non-photochemical quenching (NPQ) for all chilling-treatments in February ( and ▲) and March (○) when mango leaves were exposed to dim irradiance at each chilling temperature. *** p<0.001; # excluding data of leaves without dark-chilling pre-treatment in February (▲),” Weng et al. 2006. (Image credits: https://link.springer.com/article/10.1007/s11099-006-0015-8)
Three leaves from each of the three trees were detached and acclimated in a dark room with higher temperatures of 18-20 degrees Celsius.
These leaves were then moved to separate growth chambers that were exposed to three levels of chilling (8, 5, and 3 degrees Celsius). In addition, leaves at the same temperature were subject to the chilling for four durations (12, 4, 2, and 0 hours) and kept in the dark. In the end, they were irradiated with 20 μmol m−2 s−1 PPFD (Photosynthetic Photon Flux Density) for 1 hour in the same growth chamber.
Chlorophyll fluorescence and leaf spectral reflectance were measured before dark-chilling, 20 min after dark-chilling, end of dark-chilling, and after light-chilling.
The leaf spectral measurements were made using the CI-710 Leaf Spectrometer, manufactured by CID Bio-Science Inc. The device had a spectra-radiometer, a leaf probe, and a tungsten-halogen light source.
Chilling in the dark had no effect on PSII efficiency measured by chlorophyll fluorescence (Fv/Fm). However, even at a low irradiance of 20 μmol m−2 s−1 PPFD, chilled leaves suffered, and the PSII efficiency went down. The longer and colder temperatures had more effect on PSII efficiency.
As chlorophyll fluorescence decreased, so did PRI; see Figure 1. There was also a close relationship between chlorophyll fluorescence and NPQ. As photochemical quenching (NPQ) increases due to more xanthophyll cycling, the chlorophyll fluorescence decreases; see Figure 1.
The decrease or downregulation of PSII is a defense mechanism of the plant to protect itself from exposure to light after chilling stress. PSII efficiency in plants could overcome the chilling and irradiance effect within twenty minutes on their first exposure to light.
Influence of Temperature and Greenness of Leaves
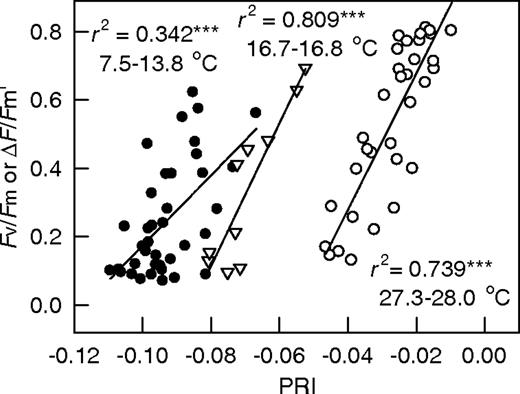
Figure 2: “Relationship between PSII efficiency (Fv/Fm or ΔF/Fm′) and PRI of mango leaves under the dark and varied intensity of sunlight outdoors. Each point represents the mean value of three leaves (replications). Symbols: ● = measured on 16 January–7 March (minimum air temperature of measuring day 7.5–13.8 °C), ▽ = 12–13 January (16.7–16.8 °C), ○ = 14–27 July (27.3–28.0 °C); *** P < 0.001,” Weng et al. 2010. (Image credits: https://doi.org/10.1093/treephys/tpq007)
Most studies that use PRI use data collected around noon. The influence of light intensity on PRI variation is well established. However, the usefulness of PRI in tracking changes in photosynthesis rate (and PSII) during a whole day or over a season was missing until a decade ago.
Several factors that influence photosynthesis and PRI had to be considered, including the following:
- Temperature
- Levels of both chlorophyll and xanthophyll
Once again, JH Weng and his associates used mango to study these changes. They chose mango because its sensitivity to low temperature could likely cause strong resultant changes in photosynthesis. Also, mango chlorophyll levels are sensitive to nitrogen deficiency.
The scientists used two-year-old potted seedlings kept outdoors. To get varying levels of chlorophyll, they were given three levels of nitrogen fertilizers (0, 1, and 2 g N) per pot, two months prior and one month prior to data collection.
Data collection was done from January to March (mid-winter to spring); and again in the hottest month of the year, July. The measurements started before sunrise at 0530 hours, and they continued once every two hours until 1900 in the evening.
All measurements were made non-destructively, using leaves attached to the seedlings. Data was collected on
- leaf color with a chlorophyll meter,
- chlorophyll fluorescence parameters with a pulse amplitude modulated fluorometer,
- leaf spectral reflectance with the portable narrow-bandwidth spectra-radiometer CI-710,
- and air temperature and light intensity.
The CI-710 Spectrometer, manufactured by CID Bio-Science Inc., has a resolution of 0.035 nm and a fiber-optic probe. Its tungsten-halogen lamp has wavelengths between 350–2000 nm, at a light intensity of 75 µmol m−2 s−1 at the leaf surface.
PRI was calculated for leaf spectral reflectance at 531 and 570 nm, using the following formula: PRI = (R531 − R570)/(R531 + R570).
Measurements of the parameters were also made under artificial light for ten days in winter and spring, under six levels of artificial illumination (0, 200, 400, 800, 1200, and 2000 μmol m−2 s−1). Also, chlorophyll and Xanthophyll levels were estimated in the laboratory.
Reacting to nitrogen levels the leaves’ color varied from green, dark green, to yellow.
At any given time of the day, both PRI and PSII efficiency decreased when light increased and were not influenced by leaf color or temperature. However, PRI varied with levels of chlorophyll or leaf color and temperature, as shown in Figure 2.
When data from all the seasons are pooled, PRI was no longer a good indicator of PSII or photosynthesis. Greener leaves had higher PRI. At pre-dawn, when temperatures were lower, the PRI was also lower, but darker leaves also had higher PRI than yellow leaves.
Similarly, PRI and xanthophyll cycle increased with increasing temperature, but greener leaves again had higher PRI values.
So, scientists developed a multiple regression model that took variations in temperature and pigment concentrations into consideration, along with light intensity. In this model, PRI was correlated and could predict PSII at various times of the day and season.
The scientists were able to recommend their model to use PRI to track dynamic changes in photosynthesis in response to season and time of the day.
From Microscopic to Regional Scales
The data collected by portable spectrometers, such as the CI-710, are making it possible for scientists to show the intricate interconnections of various physiological processes within plants that allow them to handle stress. As a result, available data and technology are able to shed light on more aspects of plant growth and health. It is fascinating that applications of spectral information covering large spatial scales have their origin at the cellular and sub-atomic levels in plants.
—
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature image courtesy of Motion Array
Sources
Latowski, D., Kuczyńska, P., & Strzałka, K. (2011). Xanthophyll cycle – a mechanism protecting plants against oxidative stress. Redox Report, 16(2), 78-90. doi:10.1179/174329211×13020951739938
Weng, J.H., Jhaung, L. H., Jiang, J. Y., Lai, G. M., & Liao, T. S. (2006). Down-regulation of photosystem 2 efficiency and spectral reflectance in mango leaves under very low irradiance and varied chilling treatments. Photosynthetica, 44(2), 248-254. doi:10.1007/s11099-006-0015-8
Weng, J. H., Jhaung, L. H., Lin, R. J., & Chen, H. Y. (2010). Relationship between photochemical efficiency of photosystem II and the photochemical reflectance index of mango tree: Merging data from different illuminations, seasons and leaf colors. Tree Physiology, 30(4), 469-478. doi:10.1093/treephys/tpq007
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- The Forest Canopy: Structure, Roles & Measurement
- Plant Respiration: Its Importance and Applications
- Forest & Plant Canopy Analysis – Tools…
- Stomatal Conductance: Functions, Measurement, and…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- Irrigating with Saline or Seawater






