March 6, 2023 at 5:00 pm | Updated March 6, 2023 at 5:00 pm | 7 min read
Roots are vital for resource acquisition and transport, determining plant growth, ability to withstand stress, and biomass accumulation. However, roots can also be affected by the environment and soil flora and fauna. Being underground, the root system, the “hidden half of plants,” has only lately been studied in detail after the development of modern tools that allow frequent examinations in the field and provide accurate results. Listed below is the most significant research on roots published in 2022, ranked according to citations.
1. Root phenotypes impact water uptake (25)
Drought is the most frequent stress crop plants encounter and limits productivity. However, little is known about the effect of soil drying on water uptake by plants. The role of stomata and transpiration in hydraulics is well-known, but not that of the root. Factors that could be important, such as species, root phenotypes, and soil types, were investigated by Cai, Ahmed, Abdalla, and Carminati to propose a hydraulic framework of soil-plant hydraulics.
They studied 11 crops and ten soil types. There was a rapid fall in soil hydraulic conductance in drying soils due to high transpiration. Water uptake was reduced when the soil water potential was between −6 to −1000 kPa. The critical soil water potential will vary depending on soil texture and root phenotypes.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Due to declining soil water potentials, root water uptake decreased faster in coarser soils. In addition, longer root lengths and hairs help postpone soil limitation in dry soils.
Environmental conditions and watering/rain will also influence water uptake by plants.
2. Root exudates can sequester soil carbon (19)
Soil carbon is one of the major carbon sinks in the world. The carbon fixed by plants and trees through photosynthesis is added to the rhizosphere as live root biomass and exudates. Microbial biomass and soil organic matter are other sources of carbon. However, the role of root exudates in forming soil organic carbon (SOC) still needs to be well studied.
Panchal, Preece, Peñuelas, and Giri, propose that root exudates in stable ecosystems such as forests and grasslands can be a significant source of SOC and lead to long-term sequestration. Moreover, it can balance the loss of SOC from agricultural lands, where SOC is depleted due to human activities. The goal can be achieved by preserving existing natural ecosystems and reforestation, afforestation, and growing grasslands. The undisturbed root systems can produce higher root exudates to build soil carbon sinks.
3. Rhizobium mediation effects on plant growth, root exudates, and metal uptake efficiency (18)
Phytoremediation to remove heavy metals polluting soils requires suitable species of plants adapted to extreme conditions and able to extract large amounts of pollutants from the ground. Metalophytes or plant hyperaccumulators usually have been found to have limited root systems, but prolific roots reaching deeper could be better suited to remove the pollutants.

Figure 1: Graphic depiction of the experiment, Sahito et al. 2022. (Image credits: https://doi.org/10.1016/j.jhazmat.2021.127442)
In this experiment, Sahito et al. establish that Rhizobium rhizogenes affect root proliferation in Sedum alfredii, a metalophyte. They also tested 20 strains of R. rhizogenes to find the influence of different strains of the bacteria on Sedum root growth and root exudate composition.
The strain AS12556 produced the highest root weight.
Five strains were short-listed in a hydroponic experiment of the bacteria’s effect on Sedum alfredii grown in varying heavy metals cadmium and zinc concentrations. Among the five strains, AS12556 proved again to be the best due to its ability to improve Sedum alfredii root proliferation, shoot biomass, lower oxidate damage, and better phytoextraction efficiency of Cd and Zn.
4. Interaction of above and below-ground plant sizes with climate (18)

Figure 2: “Raincloud plots for (a) maximum rooting depth DR and (b) maximum lateral spread LR across growth forms: forb, grass, succulent, semi-shrub, shrub, and tree. The horizontal lines in the boxplots represent the median values. The asterisk indicates the only situation where rooting depth relative to shoot volume differed between growth forms (i.e., the relative depth of forbs was significantly greater than for trees). The number at the end of each whisker indicates the total number of observations for each growth form. The maximum values for growth forms exceeding the plot scales are shown at the bottom,” Tumber‐Dávila et al. (2022). (Image credits: https://doi.org/10.1111/nph.18031).
Changes in plant size and shape above and below the ground across different climates are poorly understood. So a team of earth science researchers, Tumber-Dávila, Schenk, Du, and Jackson, assembled the most extensive global database to describe terrestrial plants’ maximum root depth, spread, and shoot size.
The researchers found that water availability and growth habit influence shoot size, and temperature seasonality influences root size. The average root system spread is twice as much as the lateral shoot growth in woody plants. So, shoot size is correlated strongly with root size. However, the climate is also an essential factor. Therefore, woody plants in arid areas have shorter shoots but deeper and narrower roots.
However, detailed information needs to be included on plant soil interactions, root depth, and lateral spread of plants at the global scale.
5. Factors underlying root architecture and function under temperature stress (17)
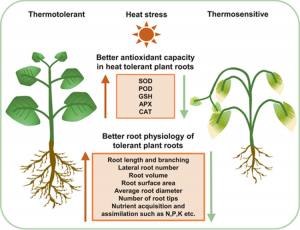
Figure 3: “Physiological and molecular changes associated with root thermotolerant and thermosensitive phenotypes, Tiwari et al., 2022. (Image credits: https://doi.org/10.1111/pce.14266)
Heat stress can significantly reduce food production. The above-ground shoot cellular, molecular, and physiological dynamics in response to heat are well understood. But our knowledge of root responses to stress or shoot-root signaling is still limited.
Tiwari, Kumar, Min, and Jagadish review the available information to present a holistic picture of root physiology, morphology, and molecular responses to a warmer climate.
Heat stress changes root cellular processes, including signaling among genes, phytohormones, reactive oxygen species, and antioxidants. Regulation and transport of hormones like auxin, cytokinin, and abscisic acid (ABA) also affect root growth and development. Epigenetic modifications by DNA methylation and acetylation influence root growth during stress.
External applications of compounds (ascorbic acids, metal ion chelators, etc.) or fungi and bacteria can reduce heat stress that can limit root biomass.
6. Root physiological adaptations enhance grain yield and nutrient use in maize (14)
Root growth and distribution adapt to the spatial availability of nutrients, such as phosphorus (P) fertilizers.
Chen, Liu, Zhao, Zhang, Ren, Li, and Wang studied the spatial distribution of nitrogen (N), phosphorus placement, and root growth to optimize yield in summer maize. They placed P at four depths 5, 10, 15, and 20 cm.
P at 15 cm depth produced higher root length density and deeper roots, leading to more N and P accumulation during growth. As a result, there was more N and P assimilation by grains leading to higher yield. As a result, the partial productivity efficiency of P and N was up by 21 percent.
So the scientists concluded that placing P at 15 cm was the best treatment. It produces more roots by reducing growth costs, having more aerenchyma and larger cortical cells, and improving nutrient absorption.
7. Root decomposition and bioremediation of contaminated soil (5)

Figure 4: PCB degradation is higher due to root decomposition than the rhizosphere, Jaing et al., 2022. (Image credits: https://doi.org/10.1016/j.soilbio.2022.108726)
Root decomposition drives microbial growth after plant harvest or death. Jiang et al. studied if decomposition influences the levels of soil organic pollutants, 2,5-dichlorobiphenyl (PCB-9) degradation, using DNA stable isotope probing (DNA-SIP). They compared the results with PCB-9 degradation in the rhizosphere.
Both treatments were faster after six weeks than the control and drove PCB degradation by changing microbial composition. But the scientists found 7.4 percent more PCB-9 degradation due to root decomposition than in the rhizosphere.
Root decomposition reduces competition between microbes. In addition, metabolites released during root decomposition favor aerobic PCB degraders and affect the entire microbial community.
Compounds associated with lipid and carbohydrate metabolism were more during root decomposition and possibly enriched PCB degraders.
8. Bacterium Herbaspirillum seropedicae alter N-dependent root growth (4)
Nitrogen fixation by root-associated bacteria can provide the roots with valuable nitrogen (N). To find how a cereal responds to the bacteria over time and N supply, a team of scientists, Kuang et al., imaged shoot and root growth and quantified N dynamics using a gnotobiotic, fabricated ecosystem. The cereal Brachypodium distachyon inoculated by N-fixing bacteria Herbaspirillum seropedicae was phenotyped to show that the bacteria changed its root and shoot growth rates, gene expression, and preference for nitrate or ammonium based on nitrogen availability.
The primary roots were longer, and root hairs were shorter at all N levels. The bacteria supplied 11 percent of the total plant N at higher N levels, but the plant N was derived from the root medium at lower N rates.
9. Root herbivory reduces arbuscular mycorrhizal fungi species (3)
Root herbivory by soil insects shapes both below and above-ground communities. Most roots are associated with arbuscular mycorrhizal (AM) fungi.
In this study, Frew examined how herbivory by Dermolepida alborhirtum can affect AM colonization of plant roots of Dichanthium sericeum and change plant nutrient uptake. The researcher found that the number of species of AM growing with the roots was reduced, and the entire AM community structure was altered. As a result, there was less phosphorus uptake in below and above-ground biomass. The study showed that underground herbivory could affect soil and plant communities.
10. Three-dimensional modeling and visualization of the rice root system (1)
Finding out rice’s root structure and spatial morphology is hindered by soil opacity. Yang et al. used a method combining dual-scale automaton and the Lindenmayer system for three-dimensional modeling and visualization to determine the factors influencing root architecture and function.
The root parameters were also calculated by destructive sampling for comparison. The new model could simulate total rice root length with an accuracy of 94.27 percent, total rice root surface area by 93.68 percent, and total rice root volume by 92.35 percent.
Maximum root width and depth were found to be correlated with measured data. Rice root length fraction, volume density, surface area density, solidity, and convex hull volume, were used to predict the relationship between root structure and functions.
Tools for Root Observation
The various studies highlight the need for more information on root responses to its environment, management, and climate. Non-destructive methods such as the CI-600 In-Situ Root Imager and CI-602 Narrow Gauge Root Imager, manufactured by CID Bio-Science Inc., can be used to image roots with minirhizotron systems over time. The devices can be inserted in root tubes to make 360 degrees scans of surrounding roots at various chosen depths. The images can be analyzed using the RootSnap! Software to estimate different root parameters. Root morphology, turnover, diseases, AM associations, and plant responses to the environment can all be studied with minirhizotron systems to help in a better understanding of below-ground processes.
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- The Forest Canopy: Structure, Roles & Measurement
- Plant Respiration: Its Importance and Applications
- Forest & Plant Canopy Analysis – Tools…
- Stomatal Conductance: Functions, Measurement, and…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- Irrigating with Saline or Seawater






