November 3, 2025 at 5:16 pm | Updated November 3, 2025 at 5:16 pm | 7 min read
- The root system architecture in plants is complex and dynamic.
- The factors that determine root form, structure, and function are plant type, root age, and environmental conditions.
- The available data is still insufficient to provide a comprehensive picture of the influence of factors on root system architecture.
The function of root system architecture has been challenging to define, especially given its underground, unseen components. The root system architecture differs across species and other factors. It is necessary to understand these factors to optimize root system architecture for agriculture, predict carbon sequestration in forests, or maintain forestry productivity.
RSA
Root system architecture (RSA) describes the shape and structure of the root system. The root architecture is not static. RSA is dynamic and complex, with a wide range of root type diversity within a single root system.
Root system architecture’s function reflects the various diversity in form and the spatial and temporal changes that occur. Three main factors influence RSA: plant type, environmental conditions, and age.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
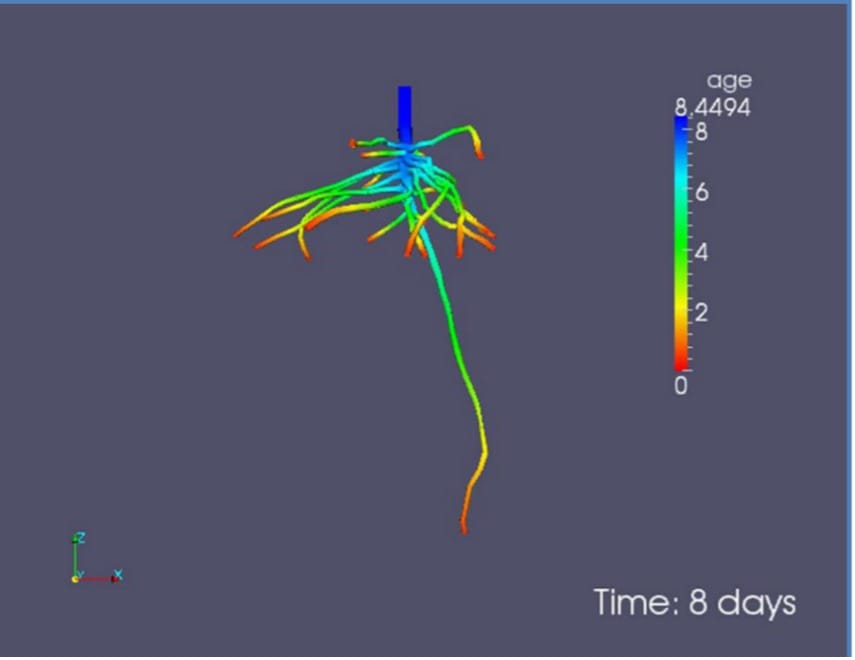
Figure 1: “Root ages distribution in the root system of Vicia faba 8 days after planting,” Vetterlein & Doussan (2016). (Image credits: DOI 10.1007/s11104-016-2849-6)
Root Age
Root growth results in an age gradient within a root system, and even within individual roots, with root tips having younger tissue (see Figure 1); therefore, root age varies with soil depth. However, nodal roots produced later will reach the same depth as pervious roots but have younger roots. Respiration rates, ion uptake, water conductance, and mucilage release vary depending on age. The impact of root age on RSA functioning is discussed below.
Root production timing
Based on ontogeny, plants grow primary, nodal/crown, and lateral roots.
- Primary roots are the first to be produced from the radicle. In taproots, this continues as the principal axis.
- Lateral roots are produced from the primary roots.
- Seminal roots grow from the area adjacent to the radicle and are common in monocots.
- Adventitious roots are formed from plant parts other than roots. For example, stem and leaf cuttings; cereals like rice, when flooded, produce nodal or crown roots from nodes.
The combination of these roots and the timing of root formation gives rise to various characteristics that also account for the differences between monocot and dicot root systems and their functions. For example, maize produces adventitious roots throughout its lifespan to repeatedly forage the same area for nutrients. While lupine, a dicot, produces a herringbone system of roots with more roots produced initially at the top and fewer later.
Size and tissue maturity
Root size and tissue maturity increase with age and are crucial for water uptake and, to some extent, for carbon transport within a plant, thereby affecting productivity.
As plants age, their root systems grow larger. The soil-root interface is thus increased, allowing the root system to explore a larger volume of soil for water and nutrients.
An increase in xylem and phloem tissue, which transport water and photosynthates, respectively, occurs due to secondary growth and expansion of the root diameter. Secondary growth is seen in dicots but not in monocots. However, even among dicots, not all roots exhibit secondary thickening. For example, only the two largest out of the six root types in Prunus spp. Undergo a root diameter increase.
Root age determines the water-uptake properties of roots, primarily because the number and size of xylem vessels involved in transporting water from the roots to the above-ground parts increase as the tissue matures over time. Hence, root axial conductance rises with age and root size. In corn roots, only metaxylem tissue is mature 25 mm beyond the tips. Still, in older tissue 25 cm from the tip, late metaxylem is also mature, and hydraulic conductance is higher.
However, radial conductivity can be hindered by apoplastic barriers, which develop due to the formation of the Casparian band and suberin lamella in the exodermis and endodermis. In perennials with longer-lived roots, there is more barrier tissue and less radial conductance. However, since the older roots constitute a larger proportion of the RSA, they contribute more to water uptake. So, it is not just the young roots that are involved in water absorption.
Older tissue closer to the trunk is more vulnerable to embolism or a gas phase in the xylem vessel due to a lowering of water potential. The primary roots are also more susceptible to embolism than lateral roots. Both dicots and monocots can repair embolism by refilling embolized vessels with water.
Mucliage is produced by young tissue at root tips and can help build a hydraulic connection between the soil and roots in the rhizosphere.
Aquaporin
Aquaporins that provide the cell-to-cell pathway for radial water conductance contribute to water uptake at 20%-90% across different species. Within a species, the difference in contribution can be 55% to 77%. Higher aquaporin expression is found in younger meristematic tissue and lower in mature tissue.
Root Senescence
As root tissue ages, the cortex cells die first. In wheat, the nuclei are lost in cells, layer by layer. Cortex death increases with tissue age and is commonly observed in annual cereals. In rice, it reduces radial water conduction. Ultimately, the root shrinks, leading to a loss of soil-root contact.
In trees and perennial plants, root age distribution varies with soil depth and during the growing season. Scientists do not believe they have the data to form a comprehensive picture of root age distribution, especially throughout the entire lifecycle of a root system, even in annuals.
Plant Type
The RSA varies across species and plant types, as they have evolved to occupy different niches and use other strategies along a gradient of nutrient availability. The primary difference in RSA is evident between monocots and dicots (Figure 2). The growth rates of the primary axes and lateral, or branching orders, differ between dicots and monocots, producing a wide range of RSA and differences in root distribution in soil for water and nutrient exploration. Root growth patterns are influenced by genetics and modified by the environment.
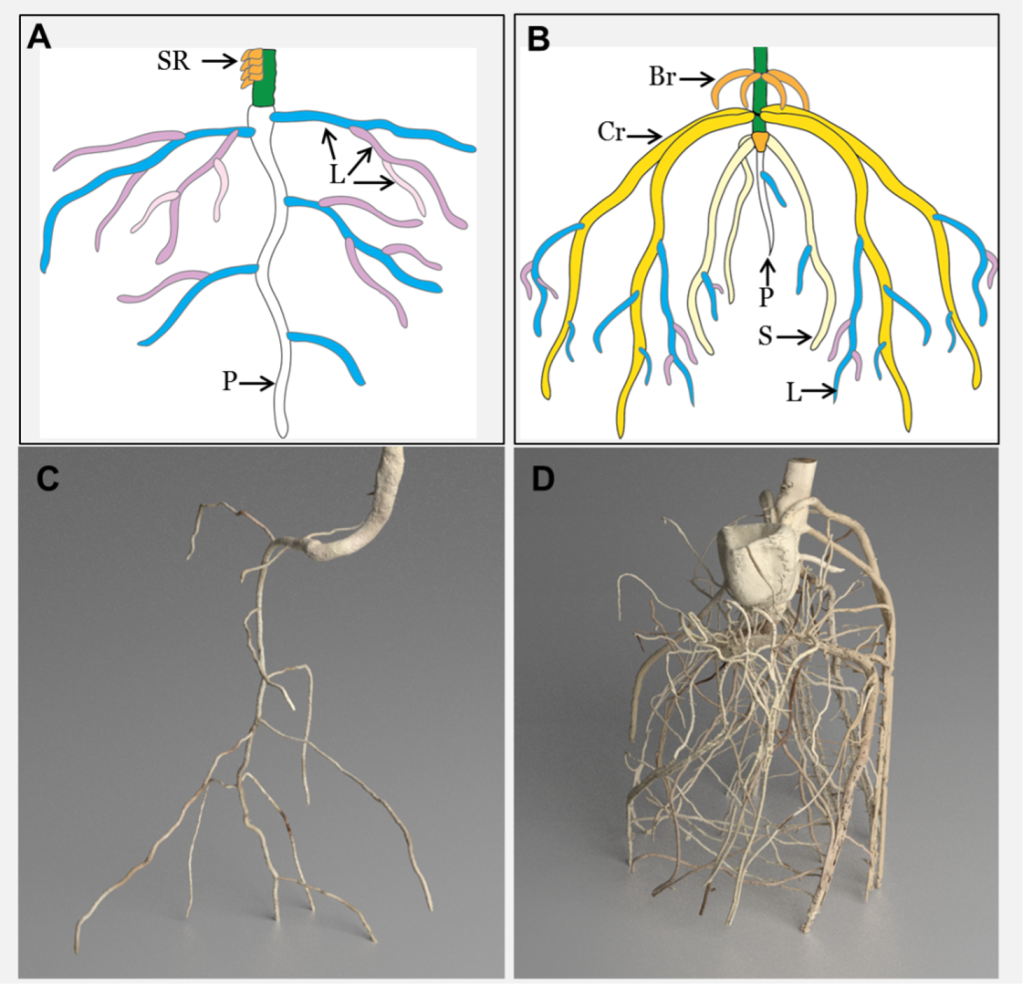
Figure 2: “Eudicot and monocot root systems. A and C represent eudicot roots, while B and D represent monocot roots. Schematic showing the dominant root types of tomato (A) and maize (B). SR = stem roots, P = primary root, L = lateral root, Br = brace root, Cr = crown root, S = seminal root (original diagram courtesy A. Rasmussen). MicroCT images of tomato (C) and maize (D) (images courtesy J. Johnson and S. Mairhofer),” Adapted from RSECO.org. (Image credits: https://www.rseco.org/content/definitions.html)
Dicots
The primary root in dicots, like trees, becomes the main tap root, and first-order laterals are formed that give rise to higher-order laterals. Secondary thickening strengthens the root system, allowing it to radiate farther from the tree trunk. In trees, the massive RSA is designed primarily for support, but in smaller plants, the RSA resulting from soil exploitation for nutrients is sufficient for anchorage.
Monocots
Grasses and annual cereals are the prominent examples of monocots. Monocots have a primary root and develop many seminal roots around it, forming a fibrous root system. Next, nodal roots grow from stem nodes and, over time, become woody, anchoring the plant and dominating the root system. Monocot RSA has no secondary thickening.
Some species, such as maize, also develop adventitious roots from stem nodes that extend into the soil for additional structural support.
Lateral and adventitious roots exhibit less gravitropism and can grow at nearly right angles to the central axis, facilitating soil exploration. Seminal roots, which are relatively few in number, play a crucial role in water uptake in maize. In comparison, nodal roots are essential for phosphorus exploration. Nitrate uptake is 1.5 times higher in the lateral than in the primary roots. At early stages, the primary root system dominates, and at later stages, it is the adventitious roots that dominate in cereals; the relative importance of the two root types varies among species and in response to different environmental conditions.
Soil and Environmental Conditions
The horizontal and vertical distribution of roots in soils that determine RSA depend on resource availability and mechanical impediments.
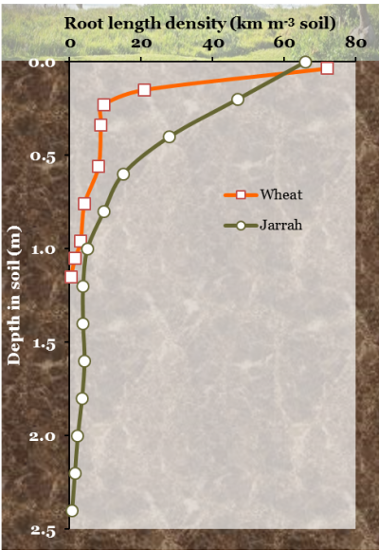
Figure 3: “Root length density in relation to depth in the soil for a wheat crop and a jarrah forest (Eucalyptus marginata),” RSECO.org. (Image credits: https://www.rseco.org/content/definitions.html)
The distribution of resources, water, and nutrients is uneven and patchy. The RSA is formed in response to resource availability, growing more roots in regions where nutrients are likely to be found.
Typically, the root length density is higher in shallower soils. Plants grow shallow roots to access phosphorus, potassium, zinc, and copper that do not migrate to deep soil levels and become bound to soil surfaces. There is more concentration of roots around the central axis as nitrogen fertilizers in crops are applied close to the plant. Monocots usually have most of their roots in shallow soils, while dicots and trees also have deeper roots. Trees also grow shallow roots to access the nutrients released by decomposing litter. The deeper roots are mainly for reaching subsoil moisture.
Younger roots absorb nutrients faster than older roots. The young roots of annual plants in their early stages enable increased nutrient uptake, thereby supporting growth. In trees and perennials, the development of fine roots from high—order laterals provides the new roots necessary for increased nutrient uptake in spring. To grow the necessary young roots in the early stages, plants allot around half the carbon fixed for root growth in annuals. Young roots can account for nearly 90% of the standing biomass in temperate trees. The necessary root expansion occurs through the development of laterals rather than the primary root.
Data Collection for RSA Studies
Data collection for RSA studies uses several approaches that combine destructive and non-destructive methods. One of the standard non-destructive methods is observing roots through transparent sections. The minirhizotron system is one such method that involves placing a transparent root tube in the soil for both short-term and long-term studies. Root growth and root changes are observed using imagers. These cameras can be inserted into the root tubes and rotated 360 degrees to scan the roots growing in the surrounding soil. The CID Bio-Science Inc. offers the CI-602 Narrow Gauge Root Imager and the CI-600 In-Situ Root Imager for scanning roots in minirhizotron systems. An accompanying software RootSnap! Detects and analyzes roots for length, width, area, and volume.
Check the two root imagers by CID Bio-Science Inc for your root research needs.
Sources
Jagodzinski, A. M., Ziółkowski, J., Warnkowska, A., & Prais, H. (2016). Tree Age Effects on Fine Root Biomass and Morphology over Chronosequences of Fagus sylvatica, Quercus robur and Alnus glutinosa Stands. PLoS ONE, 11(2), e0148668. https://doi.org/10.1371/journal.pone.0148668
Liu, Z., Marella, C. B., Hartmann, A., & Hajirezaei, M. R. (2019). An Age-Dependent Sequence of Physiological Processes Defines Developmental Root Senescence. Plant Physiology, 181(3), 993. https://doi.org/10.1104/pp.19.00809
RSECO.org. (n.d.). Plants in Action- Definitions. Retrieved from https://www.rseco.org/content/definitions.html
Tunc, C. E., & Von Wirén, N. (2025). Hidden aging: The secret role of root senescence. Trends in Plant Science, 30(5), 553-564. https://doi.org/10.1016/j.tplants.2025.02.004
Vetterlein, D., & Doussan, C. (2016). Root age distribution: how does it matter in plant processes? A focus on water uptake. Plant Soil 407, 145–160. https://doi.org/10.1007/s11104-016-2849-6
Weiser, M., Koubek, T., & Herben, T. (2016). Root Foraging Performance and Life-History Traits. Frontiers in Plant Science, 7, 779. https://doi.org/10.3389/fpls.2016.00779
Wells, C.E., & Eissenstat, D.M. (2003) Beyond the roots of young seedlings: the influence of age and order on fine root physiology. J Plant Growth Regul 21(4):324–334
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- Plant Respiration: Its Importance and Applications
- The Forest Canopy: Structure, Roles & Measurement
- Stomatal Conductance: Functions, Measurement, and…
- Forest & Plant Canopy Analysis – Tools…
- Root Respiration: Importance and Applications
- The Importance of Leaf Area Index (LAI) in…
- 50 Best Universities for Plant Science






